Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biocompatibility and biodegradation of poly(lactic acid) (PLA) and an immiscible PLA/poly(ε-caprolactone) (PCL) blend compatibilized by poly(ε-caprolactone-b-tetrahydrofuran) implanted in horses
Polymer Journal ( IF 2.8 ) Pub Date : 2020-01-28 , DOI: 10.1038/s41428-020-0308-y Júlia R. G. Carvalho , Gabriel Conde , Marina L. Antonioli , Paula P. Dias , Rosemeri O. Vasconcelos , Sebastião R. Taboga , Paulo A. Canola , Marcelo A. Chinelatto , Gener T. Pereira , Guilherme C. Ferraz
Polymer Journal ( IF 2.8 ) Pub Date : 2020-01-28 , DOI: 10.1038/s41428-020-0308-y Júlia R. G. Carvalho , Gabriel Conde , Marina L. Antonioli , Paula P. Dias , Rosemeri O. Vasconcelos , Sebastião R. Taboga , Paulo A. Canola , Marcelo A. Chinelatto , Gener T. Pereira , Guilherme C. Ferraz
|
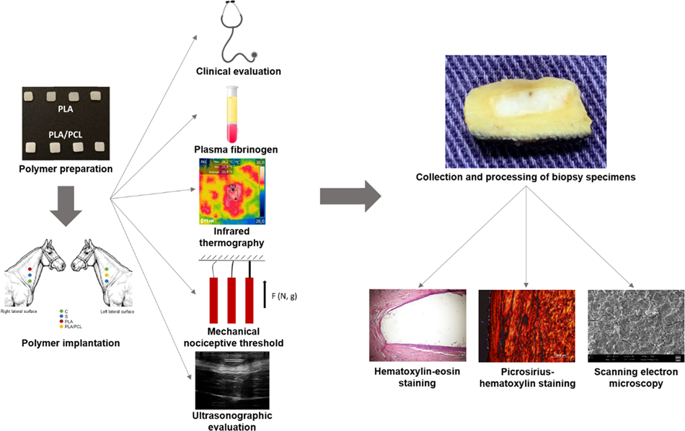
This paper focuses on the biocompatibility and biodegradation of PLA and a recently developed PLA/PCL blend containing an in vitro nontoxic compatibilizer based on a low-molecular-weight triblock copolymer derived from ε-caprolactone and tetrahydrofuran. The polymers were implanted subcutaneously in the lateral surface of the neck of horses. Physical examination, plasma fibrinogen (PF) analysis, infrared thermography (IT), mechanical nociceptive threshold (MNT) analysis, and ultrasonography were performed. After 24 weeks, the biomaterials were removed for histochemical analysis using hematoxylin-eosin (HE) and picrosirius-hematoxylin (PSH) staining. Scanning electron microscopy (SEM) was employed to determine changes in the surface morphology of the PLA and PLA/PCL blend. There were no clinical or PF changes. IT indicated a transient increase in cutaneous temperature (CT), while MNT decreased after the procedure in both the implanted groups. Ultrasonography revealed edema after the procedure and the loss of echogenicity of the polymers after implantation. Both polymers elicited a foreign body response under microscopic analysis. The PSH technique revealed a fibrotic reaction with collagen deposition around the polymers. SEM showed surface roughness, suggesting a biodegradation process. In conclusion, PLA and the PLA/PCL blend were biocompatible and biodegradable, with potential for use in equine medicine. We evaluated the biocompatibility and biodegradability of implants made of pure PLA and a PLA/PCL blend compatibilized with poly(ɛ-caprolactone-b-tetrahydrofuran) in horses by physical examination, plasma fibrinogen, thermographic, mechanical nociceptive threshold, and ultrasound tests. We also conducted histopathological and surface morphology analyses. Pure PLA and PLA/PCL blends subcutaneously implanted stimulated a minimal inflammatory response and supported cellular infiltration, being biocompatible and biodegradable in horses, with potential for use in equine medicine.
中文翻译:

聚(乳酸)(PLA)和不混溶的 PLA/聚(ε-己内酯)(PCL)共混物的生物相容性和生物降解由植入马的聚(ε-己内酯-b-四氢呋喃)相容
本文重点研究 PLA 和最近开发的 PLA/PCL 混合物的生物相容性和生物降解性,该混合物含有基于衍生自 ε-己内酯和四氢呋喃的低分子量三嵌段共聚物的体外无毒增容剂。将聚合物皮下植入马颈的侧面。进行了体格检查、血浆纤维蛋白原 (PF) 分析、红外热成像 (IT)、机械痛觉阈值 (MNT) 分析和超声检查。24 周后,取出生物材料,使用苏木精-伊红 (HE) 和天狼星-苏木精 (PSH) 染色进行组织化学分析。使用扫描电子显微镜 (SEM) 来确定 PLA 和 PLA/PCL 共混物的表面形态变化。没有临床或 PF 变化。IT 表明皮肤温度 (CT) 短暂升高,而两个植入组在手术后 MNT 均下降。超声检查显示手术后水肿和植入后聚合物回声丧失。在显微分析下,两种聚合物均引起异物反应。PSH 技术揭示了聚合物周围胶原蛋白沉积的纤维化反应。SEM 显示表面粗糙,表明存在生物降解过程。总之,PLA 和 PLA/PCL 混合物具有生物相容性和可生物降解性,具有用于马医学的潜力。我们通过体格检查、血浆纤维蛋白原、热成像评估了由纯 PLA 和与聚(ɛ-己内酯-b-四氢呋喃)相容的 PLA/PCL 混合物制成的植入物的生物相容性和生物降解性,机械痛觉阈值和超声检查。我们还进行了组织病理学和表面形态学分析。皮下植入的纯 PLA 和 PLA/PCL 混合物刺激了最小的炎症反应并支持细胞浸润,在马体内具有生物相容性和生物可降解性,具有用于马医学的潜力。
更新日期:2020-01-28
中文翻译:

聚(乳酸)(PLA)和不混溶的 PLA/聚(ε-己内酯)(PCL)共混物的生物相容性和生物降解由植入马的聚(ε-己内酯-b-四氢呋喃)相容
本文重点研究 PLA 和最近开发的 PLA/PCL 混合物的生物相容性和生物降解性,该混合物含有基于衍生自 ε-己内酯和四氢呋喃的低分子量三嵌段共聚物的体外无毒增容剂。将聚合物皮下植入马颈的侧面。进行了体格检查、血浆纤维蛋白原 (PF) 分析、红外热成像 (IT)、机械痛觉阈值 (MNT) 分析和超声检查。24 周后,取出生物材料,使用苏木精-伊红 (HE) 和天狼星-苏木精 (PSH) 染色进行组织化学分析。使用扫描电子显微镜 (SEM) 来确定 PLA 和 PLA/PCL 共混物的表面形态变化。没有临床或 PF 变化。IT 表明皮肤温度 (CT) 短暂升高,而两个植入组在手术后 MNT 均下降。超声检查显示手术后水肿和植入后聚合物回声丧失。在显微分析下,两种聚合物均引起异物反应。PSH 技术揭示了聚合物周围胶原蛋白沉积的纤维化反应。SEM 显示表面粗糙,表明存在生物降解过程。总之,PLA 和 PLA/PCL 混合物具有生物相容性和可生物降解性,具有用于马医学的潜力。我们通过体格检查、血浆纤维蛋白原、热成像评估了由纯 PLA 和与聚(ɛ-己内酯-b-四氢呋喃)相容的 PLA/PCL 混合物制成的植入物的生物相容性和生物降解性,机械痛觉阈值和超声检查。我们还进行了组织病理学和表面形态学分析。皮下植入的纯 PLA 和 PLA/PCL 混合物刺激了最小的炎症反应并支持细胞浸润,在马体内具有生物相容性和生物可降解性,具有用于马医学的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号