当前位置:
X-MOL 学术
›
Mol. Cell. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular dissection of TNFR-TNFα bidirectional signaling reveals both cooperative and antagonistic interactions with p75 neurotrophic factor receptor in axon patterning.
Molecular and Cellular Neuroscience ( IF 3.5 ) Pub Date : 2020-01-28 , DOI: 10.1016/j.mcn.2020.103467 K D Kuhn 1 , K Edamura 1 , N Bhatia 1 , I Cheng 2 , S A Clark 2 , C V Haynes 1 , D L Heffner 1 , F Kabir 1 , J Velasquez 3 , A J Spano 1 , C D Deppmann 4 , A B Keeler 1
Molecular and Cellular Neuroscience ( IF 3.5 ) Pub Date : 2020-01-28 , DOI: 10.1016/j.mcn.2020.103467 K D Kuhn 1 , K Edamura 1 , N Bhatia 1 , I Cheng 2 , S A Clark 2 , C V Haynes 1 , D L Heffner 1 , F Kabir 1 , J Velasquez 3 , A J Spano 1 , C D Deppmann 4 , A B Keeler 1
Affiliation
|
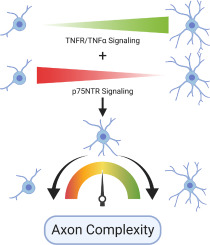
During neural development, complex organisms rely on progressive and regressive events whereby axons, synapses, and neurons are overproduced followed by selective elimination of a portion of these components. Tumor necrosis factor α (TNFα) together with its cognate receptor (Tumor necrosis factor receptor 1; TNFR1) have been shown to play both regressive (i.e. forward signaling from the receptor) and progressive (i.e. reverse signaling from the ligand) roles in sympathetic neuron development. In contrast, a paralog of TNFR1, p75 neurotrophic factor receptor (p75NTR) promotes mainly regressive developmental events in sympathetic neurons. Here we examine the interplay between these paralogous receptors in the regulation of axon branch elimination and arborization. We confirm previous reports that these TNFR1 family members are individually capable of promoting ligand-dependent suppression of axon growth and branching. Remarkably, p75NTR and TNFR1 physically interact and p75NTR requires TNFR1 for ligand-dependent axon suppression of axon branching but not vice versa. We also find that p75NTR forward signaling and TNFα reverse signaling are functionally antagonistic. Finally, we find that TNFα reverse signaling is necessary for nerve growth factor (NGF) dependent axon growth. Taken together these findings demonstrate several levels of synergistic and antagonistic interactions using very few signaling pathways and that the balance of these synergizing and opposing signals act to ensure proper axon growth and patterning.
中文翻译:

TNFR-TNFα双向信号的分子解剖揭示了轴突模式中与p75神经营养因子受体的协同和拮抗作用。
在神经发育过程中,复杂的生物体依赖于进行性和回归性事件,从而轴突,突触和神经元被过度生产,然后选择性消除其中的一部分。肿瘤坏死因子α(TNFα)及其同源受体(肿瘤坏死因子受体1; TNFR1)已显示出在交感神经元中既具有回归(即来自受体的正向信号传导)又具有进行性(即来自配体的反向信号)作用。发展。相比之下,TNFR1,p75神经营养因子受体(p75NTR)的旁系同源物主要促进交感神经元的退行性发育。在这里,我们检查在轴突分支消除和乔木调节这些旁系受体之间的相互作用。我们确认以前的报告,这些TNFR1家族成员个别能够促进轴突生长和分支的配体依赖性抑制。值得注意的是,p75NTR和TNFR1在物理上相互作用,而p75NTR需要TNFR1来抑制配体依赖性轴突对轴突分支的抑制,反之则不然。我们还发现p75NTR正向信号传导和TNFα反向信号传导在功能上是拮抗的。最后,我们发现TNFα反向信号对于神经生长因子(NGF)依赖的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。p75NTR和TNFR1发生物理相互作用,p75NTR需要TNFR1抑制配体依赖性轴突对轴突分支的抑制,反之则不然。我们还发现p75NTR正向信号传导和TNFα反向信号传导在功能上是拮抗的。最后,我们发现TNFα反向信号对于神经生长因子(NGF)依赖的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。p75NTR和TNFR1发生物理相互作用,p75NTR需要TNFR1抑制配体依赖性轴突对轴突分支的抑制,反之则不然。我们还发现p75NTR正向信号传导和TNFα反向信号传导在功能上是拮抗的。最后,我们发现TNFα反向信号对于神经生长因子(NGF)依赖的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。我们发现TNFα反向信号对于依赖神经生长因子(NGF)的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。我们发现TNFα反向信号对于依赖神经生长因子(NGF)的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。
更新日期:2020-01-30
中文翻译:

TNFR-TNFα双向信号的分子解剖揭示了轴突模式中与p75神经营养因子受体的协同和拮抗作用。
在神经发育过程中,复杂的生物体依赖于进行性和回归性事件,从而轴突,突触和神经元被过度生产,然后选择性消除其中的一部分。肿瘤坏死因子α(TNFα)及其同源受体(肿瘤坏死因子受体1; TNFR1)已显示出在交感神经元中既具有回归(即来自受体的正向信号传导)又具有进行性(即来自配体的反向信号)作用。发展。相比之下,TNFR1,p75神经营养因子受体(p75NTR)的旁系同源物主要促进交感神经元的退行性发育。在这里,我们检查在轴突分支消除和乔木调节这些旁系受体之间的相互作用。我们确认以前的报告,这些TNFR1家族成员个别能够促进轴突生长和分支的配体依赖性抑制。值得注意的是,p75NTR和TNFR1在物理上相互作用,而p75NTR需要TNFR1来抑制配体依赖性轴突对轴突分支的抑制,反之则不然。我们还发现p75NTR正向信号传导和TNFα反向信号传导在功能上是拮抗的。最后,我们发现TNFα反向信号对于神经生长因子(NGF)依赖的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。p75NTR和TNFR1发生物理相互作用,p75NTR需要TNFR1抑制配体依赖性轴突对轴突分支的抑制,反之则不然。我们还发现p75NTR正向信号传导和TNFα反向信号传导在功能上是拮抗的。最后,我们发现TNFα反向信号对于神经生长因子(NGF)依赖的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。p75NTR和TNFR1发生物理相互作用,p75NTR需要TNFR1抑制配体依赖性轴突对轴突分支的抑制,反之则不然。我们还发现p75NTR正向信号传导和TNFα反向信号传导在功能上是拮抗的。最后,我们发现TNFα反向信号对于神经生长因子(NGF)依赖的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。我们发现TNFα反向信号对于依赖神经生长因子(NGF)的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。我们发现TNFα反向信号对于依赖神经生长因子(NGF)的轴突生长是必需的。综上所述,这些发现证明了使用极少的信号传导途径的协同和拮抗相互作用的几种水平,并且这些协同和相反信号的平衡起到确保适当的轴突生长和构图的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号