当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic degradation of ciprofloxacin by a visible-light-assisted peroxymonosulfate activation system: Performance and mechanism.
Water Research ( IF 12.8 ) Pub Date : 2020-01-28 , DOI: 10.1016/j.watres.2020.115559 Fei Chen 1 , Gui-Xiang Huang 1 , Fu-Bing Yao 2 , Qi Yang 2 , Yu-Ming Zheng 3 , Quan-Bao Zhao 3 , Han-Qing Yu 4
Water Research ( IF 12.8 ) Pub Date : 2020-01-28 , DOI: 10.1016/j.watres.2020.115559 Fei Chen 1 , Gui-Xiang Huang 1 , Fu-Bing Yao 2 , Qi Yang 2 , Yu-Ming Zheng 3 , Quan-Bao Zhao 3 , Han-Qing Yu 4
Affiliation
|
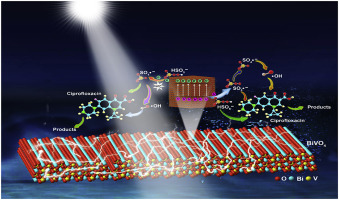
Peroxymonosulfate (PMS) is extensively used as an oxidant to develop the sulfate radical-based advanced oxidation processes in the decontamination of organic pollutants and various PMS activation methods have been explored. Visible-light-assisted PMS activation to construct a Fenton-like process has shown a great potential for pollution control. In our work, BiVO4 nanosheets were prepared using a hydrothermal process and used to activate PMS under visible light. A rapid degradation of ciprofloxacin (CIP) was achieved by dosing PMS (0.96 g/L), BiVO4 (0.32 g/L) under visible light with a reaction rate constant of 77.72-fold higher than that in the BiVO4/visible light process. The electron spin resonance and free radical quenching experiments indicate that reactive species of •O2-, h+, •OH and SO4•- all worked, where h+, •OH and SO4•- were found as the dominant contributors to the CIP degradation. The spectroscopic analyses further demonstrate that the photoinduced electrons were directly involved in the PMS activation process. The generated •O2- was partially utilized to activate PMS and more •OH was produced because of the chain reactions between SO4•- and H2O/OH-. In this process, PMS acted as an electron acceptor to transfer the photo-induced charges from the conduction band of BiVO4 and PMS was successfully activated to yield the high-powered oxidative species. From the degradation intermediates of CIP detected by a liquid-chromatography-mass spectrometer, the possible degradation pathways were proposed. The substantially decreased toxicity of CIP after the reaction was also observed. This work might provide new insights into the visible-light-assisted PMS activation mechanisms and is useful to construct environmentally-friendly catalytic processes for the efficient degradation of organic pollutants.
中文翻译:

可见光辅助过一硫酸盐活化系统催化降解环丙沙星:性能和机理。
过氧一硫酸盐(PMS)被广泛用作氧化剂,以发展基于硫酸根的高级氧化过程,用于有机污染物的去污,并且已经探索了各种PMS活化方法。可见光辅助的PMS活化以构建类似Fenton的过程已显示出巨大的污染控制潜力。在我们的工作中,使用水热工艺制备了BiVO4纳米片,并用于在可见光下激活PMS。通过在可见光下投加PMS(0.96 g / L),BiVO4(0.32 g / L),可实现环丙沙星(CIP)的快速降解,其反应速率常数比BiVO4 /可见光工艺高77.72倍。电子自旋共振和自由基猝灭实验表明,•O2-,h +,•OH和SO4•-的反应性均起作用,其中h +,发现•OH和SO4•-是CIP降解的主要因素。光谱分析进一步表明,光致电子直接参与了PMS活化过程。生成的•O2-被部分用于激活PMS,并且由于SO4•-和H2O / OH-之间的链反应而生成了更多的•OH。在此过程中,PMS充当电子受体,从BiVO4的导带转移光诱导的电荷,并且成功激活PMS以产生高功率的氧化物质。从液相色谱-质谱仪检测到的CIP降解中间体中,提出了可能的降解途径。还观察到反应后CIP的毒性大大降低。
更新日期:2020-01-29
中文翻译:

可见光辅助过一硫酸盐活化系统催化降解环丙沙星:性能和机理。
过氧一硫酸盐(PMS)被广泛用作氧化剂,以发展基于硫酸根的高级氧化过程,用于有机污染物的去污,并且已经探索了各种PMS活化方法。可见光辅助的PMS活化以构建类似Fenton的过程已显示出巨大的污染控制潜力。在我们的工作中,使用水热工艺制备了BiVO4纳米片,并用于在可见光下激活PMS。通过在可见光下投加PMS(0.96 g / L),BiVO4(0.32 g / L),可实现环丙沙星(CIP)的快速降解,其反应速率常数比BiVO4 /可见光工艺高77.72倍。电子自旋共振和自由基猝灭实验表明,•O2-,h +,•OH和SO4•-的反应性均起作用,其中h +,发现•OH和SO4•-是CIP降解的主要因素。光谱分析进一步表明,光致电子直接参与了PMS活化过程。生成的•O2-被部分用于激活PMS,并且由于SO4•-和H2O / OH-之间的链反应而生成了更多的•OH。在此过程中,PMS充当电子受体,从BiVO4的导带转移光诱导的电荷,并且成功激活PMS以产生高功率的氧化物质。从液相色谱-质谱仪检测到的CIP降解中间体中,提出了可能的降解途径。还观察到反应后CIP的毒性大大降低。


























 京公网安备 11010802027423号
京公网安备 11010802027423号