当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Altering the Electrochemical Pathway of Sulfur Chemistry with Oxygen for High Energy Density and Low Shuttling in a Na/S Battery
ACS Energy Letters ( IF 22.0 ) Pub Date : 2020-01-29 , DOI: 10.1021/acsenergylett.9b02746 Sanpei Zhang , Travis P. Pollard 1 , Xu Feng , Oleg Borodin 1 , Kang Xu 1 , Zheng Li
ACS Energy Letters ( IF 22.0 ) Pub Date : 2020-01-29 , DOI: 10.1021/acsenergylett.9b02746 Sanpei Zhang , Travis P. Pollard 1 , Xu Feng , Oleg Borodin 1 , Kang Xu 1 , Zheng Li
Affiliation
|
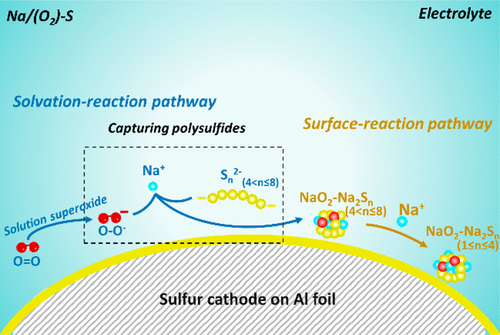
In this work, we demonstrate that intrinsically altering the reaction pathway of a sulfur-based cathode with designed additional redox activities could simultaneously suppress polysulfide shuttling and enhance energy density. A new hybrid sulfur–oxygen chemistry was described for room-temperature Na/S batteries, where the solvated sodium–oxygen reaction in the electrolyte redirected the cathode chemistry via the formation of NaO2–Na2Sn (1< n ≤ 4) clusters at the nanoscale. These intermediate oxy-sulfur species serve as an effective mediator to immobilize the polysulfide species and unlock high specific capacity from the hybrid cathode. This new cathode chemistry delivers a high reversible capacity of over 1400 mA h/g, low overpotential (∼250 mV), and stable cycling performance (over 800 mA h/g after 50 cycles). The judicious hybridization of oxygen and sulfur chemistries has resolved the persistent degradation that has been plaguing all sulfur-based cathodes and enabled a high energy and reversible Na/S battery at room temperature.
中文翻译:

在Na / S电池中用氧气改变硫化学的电化学途径以实现高能量密度和低穿梭
在这项工作中,我们证明本质上改变具有设计的其他氧化还原活性的硫基阴极的反应途径可以同时抑制多硫化物的穿梭并提高能量密度。描述了一种用于室温Na / S电池的新的混合硫-氧化学,其中电解质中的溶剂化钠-氧反应通过形成NaO 2 -Na 2 S n(1 < n≤4)在纳米级成簇。这些中间的氧-硫物质充当固定多硫化物物质并从混合阴极释放高比容量的有效介体。这种新的阴极化学物质可提供超过1400 mA h / g的高可逆容量,低过电势(〜250 mV)和稳定的循环性能(50个循环后超过800 mA h / g)。氧气和硫化学物质的明智杂交解决了困扰所有硫基阴极的持续降解问题,并在室温下实现了高能量和可逆的Na / S电池。
更新日期:2020-01-29
中文翻译:

在Na / S电池中用氧气改变硫化学的电化学途径以实现高能量密度和低穿梭
在这项工作中,我们证明本质上改变具有设计的其他氧化还原活性的硫基阴极的反应途径可以同时抑制多硫化物的穿梭并提高能量密度。描述了一种用于室温Na / S电池的新的混合硫-氧化学,其中电解质中的溶剂化钠-氧反应通过形成NaO 2 -Na 2 S n(1 < n≤4)在纳米级成簇。这些中间的氧-硫物质充当固定多硫化物物质并从混合阴极释放高比容量的有效介体。这种新的阴极化学物质可提供超过1400 mA h / g的高可逆容量,低过电势(〜250 mV)和稳定的循环性能(50个循环后超过800 mA h / g)。氧气和硫化学物质的明智杂交解决了困扰所有硫基阴极的持续降解问题,并在室温下实现了高能量和可逆的Na / S电池。



























 京公网安备 11010802027423号
京公网安备 11010802027423号