当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substitutions of Amino Acid Residues in the Substrate Binding Site of Horse Liver Alcohol Dehydrogenase Have Small Effects on the Structures but Significantly Affect Catalysis of Hydrogen Transfer.
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-10 , DOI: 10.1021/acs.biochem.9b01074 Keehyuk Kim 1 , Bryce V Plapp 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-10 , DOI: 10.1021/acs.biochem.9b01074 Keehyuk Kim 1 , Bryce V Plapp 1
Affiliation
|
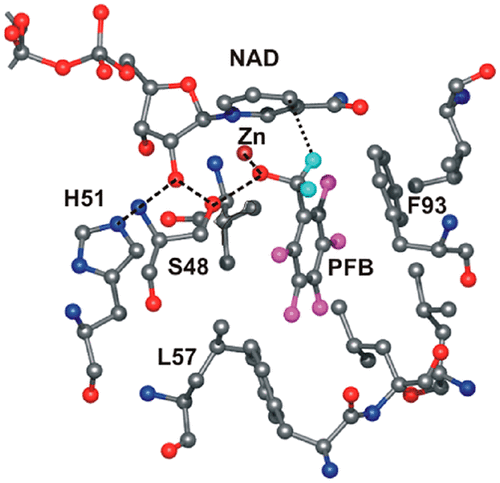
Previous studies showed that the L57F and F93W alcohol dehydrogenases catalyze the oxidation of benzyl alcohol with some quantum mechanical hydrogen tunneling, whereas the V203A enzyme has diminished tunneling. Here, steady-state kinetics for the L57F and F93W enzymes were studied, and microscopic rate constants for the ordered bi-bi mechanism were estimated from simulations of transient kinetics for the S48T, F93A, S48T/F93A, F93W, and L57F enzymes. Catalytic efficiencies for benzyl alcohol oxidation (V1/EtKb) vary over a range of ∼100-fold for the less active enzymes up to the L57F enzyme and are mostly associated with the binding of alcohol rather than the rate constants for hydride transfer. In contrast, catalytic efficiencies for benzaldehyde reduction (V2/EtKp) are ∼500-fold higher for the L57F enzyme than for the less active enzymes and are mostly associated with the rate constants for hydride transfer. Atomic-resolution structures (1.1 Å) for the F93W and L57F enzymes complexed with NAD+ and 2,3,4,5,6-pentafluorobenzyl alcohol or 2,2,2-trifluoroethanol are almost identical to previous structures for the wild-type, S48T, and V203A enzymes. Least-squares refinement with SHELXL shows that the nicotinamide ring is slightly strained in all complexes and that the apparent donor–acceptor distances from C4N of NAD to C7 of pentafluorobenzyl alcohol range from 3.28 to 3.49 Å (±0.02 Å) and are not correlated with the rate constants for hydride transfer or hydrogen tunneling. How the substitutions affect the dynamics of reorganization during hydrogen transfer and the extent of tunneling remain to be determined.
中文翻译:

马肝酒精脱氢酶底物结合位点的氨基酸残基取代对结构的影响很小,但对氢转移的催化作用有显着影响。
先前的研究表明,L57F和F93W醇脱氢酶通过一些量子机械氢隧穿来催化苯甲醇的氧化,而V203A酶则减少了隧穿。在这里,我们研究了L57F和F93W酶的稳态动力学,并通过对S48T,F93A,S48T / F93A,F93W和L57F酶的瞬态动力学进行了仿真,估算了有序双向Bi机制的微观速率常数。苯甲醇氧化的催化效率(V 1 / E t K b对于活性较低的酶(直至L57F酶),在大约100倍的范围内变化,并且大多数与醇的结合有关,而不是与氢化物转移的速率常数有关。相反,L57F酶的苯甲醛还原催化效率(V 2 / E t K p)比活性较低的酶高约500倍,并且主要与氢化物转移的速率常数有关。与NAD +复合的F93W和L57F酶的原子分辨结构(1.1Å)2,3,4,5,6-五氟苄醇或2,2,2-三氟乙醇与野生型,S48T和V203A酶的先前结构几乎相同。用SHELXL进行的最小二乘修正表明,烟酰胺环在所有络合物中都略微拉紧,从NAD的C4N到五氟苄醇的C7的表观供体-受体距离在3.28至3.49Å(±0.02Å)之间,与氢化物转移或氢隧穿的速率常数。取代如何影响氢转移过程中的重组动力学和隧穿程度仍有待确定。
更新日期:2020-02-10
中文翻译:

马肝酒精脱氢酶底物结合位点的氨基酸残基取代对结构的影响很小,但对氢转移的催化作用有显着影响。
先前的研究表明,L57F和F93W醇脱氢酶通过一些量子机械氢隧穿来催化苯甲醇的氧化,而V203A酶则减少了隧穿。在这里,我们研究了L57F和F93W酶的稳态动力学,并通过对S48T,F93A,S48T / F93A,F93W和L57F酶的瞬态动力学进行了仿真,估算了有序双向Bi机制的微观速率常数。苯甲醇氧化的催化效率(V 1 / E t K b对于活性较低的酶(直至L57F酶),在大约100倍的范围内变化,并且大多数与醇的结合有关,而不是与氢化物转移的速率常数有关。相反,L57F酶的苯甲醛还原催化效率(V 2 / E t K p)比活性较低的酶高约500倍,并且主要与氢化物转移的速率常数有关。与NAD +复合的F93W和L57F酶的原子分辨结构(1.1Å)2,3,4,5,6-五氟苄醇或2,2,2-三氟乙醇与野生型,S48T和V203A酶的先前结构几乎相同。用SHELXL进行的最小二乘修正表明,烟酰胺环在所有络合物中都略微拉紧,从NAD的C4N到五氟苄醇的C7的表观供体-受体距离在3.28至3.49Å(±0.02Å)之间,与氢化物转移或氢隧穿的速率常数。取代如何影响氢转移过程中的重组动力学和隧穿程度仍有待确定。



























 京公网安备 11010802027423号
京公网安备 11010802027423号