当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapid, two-pot procedure for the synthesis of dihydropyridinones; total synthesis of aza-goniothalamin.
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-01-28 , DOI: 10.3762/bjoc.16.15 Thomas J Cogswell 1 , Craig S Donald 2 , Rodolfo Marquez 1, 3
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-01-28 , DOI: 10.3762/bjoc.16.15 Thomas J Cogswell 1 , Craig S Donald 2 , Rodolfo Marquez 1, 3
Affiliation
|
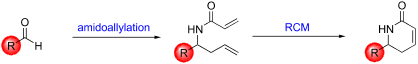
A fast, protecting-group-free synthesis of dihydropyridinones has been developed. Starting from commercially available aldehydes, a novel one-pot amidoallylation gave access to diene compounds in good yields. Ring-closing metathesis conditions were then employed to produce the target dihydropyridinones efficiently and in high yields.
中文翻译:

快速的两锅法合成二氢吡啶并酮;全合成氮杂goniothalamin。
已经开发了快速,无保护基的二氢吡啶并酮的合成。从可商购获得的醛开始,新型的一锅式酰胺基化可以高收率获得二烯化合物。然后采用闭环复分解条件有效且高产率地生产目标二氢吡啶并酮。
更新日期:2020-01-29
中文翻译:

快速的两锅法合成二氢吡啶并酮;全合成氮杂goniothalamin。
已经开发了快速,无保护基的二氢吡啶并酮的合成。从可商购获得的醛开始,新型的一锅式酰胺基化可以高收率获得二烯化合物。然后采用闭环复分解条件有效且高产率地生产目标二氢吡啶并酮。


























 京公网安备 11010802027423号
京公网安备 11010802027423号