当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Human Serum Albumin Binding in a Vial: A Novel UV-pH Titration Method To Assist Drug Design.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-02-11 , DOI: 10.1021/acs.jmedchem.0c00046 Gergő Dargó 1, 2 , Dávid Bajusz 3 , Kristóf Simon 4 , Judit Müller 5 , György T Balogh 1, 2, 6
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-02-11 , DOI: 10.1021/acs.jmedchem.0c00046 Gergő Dargó 1, 2 , Dávid Bajusz 3 , Kristóf Simon 4 , Judit Müller 5 , György T Balogh 1, 2, 6
Affiliation
|
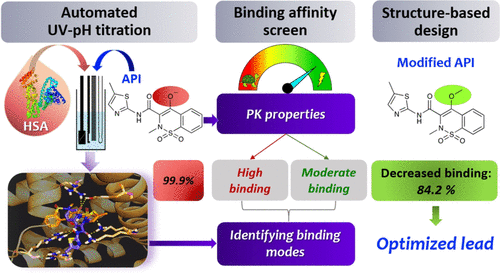
The knowledge on human serum albumin (HSA) binding is of utmost importance as it affects pharmacokinetic behavior and bioavailability of drugs. In this article, we report a novel method to screen for ionizable molecules with high HSA binding affinity based on pKa shifts using UV-pH titration. We investigated the HSA binding of 27 drugs and compared the results to experimental data from conventional methods. In most cases, significant shifts (ΔpKa > 0.1) were observed for drugs with high HSA binding, while no change could be detected for low-affinity binders. We showed the pivotal role of ionization centers in the formation of strong interactions between drug and HSA using molecular docking studies. We also verified our findings by testing five modified analogues designed by structural considerations. Significant decreases in their HSA binding proved that the UV-pH titration method combined with an in silico support can be used as a medicinal chemistry tool to assist rational molecular design.
中文翻译:

小瓶中的人血清白蛋白结合:一种新型的UV-pH滴定法来辅助药物设计。
关于人血清白蛋白(HSA)结合的知识至关重要,因为它会影响药物的药代动力学行为和生物利用度。在本文中,我们报告了一种新颖的方法,该方法可使用UV-pH滴定基于pKa位移来筛选具有高HSA结合亲和力的可电离分子。我们研究了27种药物的HSA结合,并将结果与常规方法的实验数据进行了比较。在大多数情况下,对于具有高HSA结合力的药物,观察到了显着变化(ΔpKa> 0.1),而对于低亲和力结合剂,则未检测到变化。我们使用分子对接研究显示了电离中心在药物与HSA之间形成强相互作用的关键作用。我们还通过测试了五种经过结构考量设计的修饰类似物来验证我们的发现。
更新日期:2020-02-11
中文翻译:

小瓶中的人血清白蛋白结合:一种新型的UV-pH滴定法来辅助药物设计。
关于人血清白蛋白(HSA)结合的知识至关重要,因为它会影响药物的药代动力学行为和生物利用度。在本文中,我们报告了一种新颖的方法,该方法可使用UV-pH滴定基于pKa位移来筛选具有高HSA结合亲和力的可电离分子。我们研究了27种药物的HSA结合,并将结果与常规方法的实验数据进行了比较。在大多数情况下,对于具有高HSA结合力的药物,观察到了显着变化(ΔpKa> 0.1),而对于低亲和力结合剂,则未检测到变化。我们使用分子对接研究显示了电离中心在药物与HSA之间形成强相互作用的关键作用。我们还通过测试了五种经过结构考量设计的修饰类似物来验证我们的发现。



























 京公网安备 11010802027423号
京公网安备 11010802027423号