当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Formal Synthesis of MDM2 Antagonist RG7388 and Its Analogues
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-03-10 , DOI: 10.1002/cjoc.201900530 Xue‐Jie Zou 1 , Wu‐Lin Yang 1 , Jing‐Yan Zhu 1 , Wei‐Ping Deng 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-03-10 , DOI: 10.1002/cjoc.201900530 Xue‐Jie Zou 1 , Wu‐Lin Yang 1 , Jing‐Yan Zhu 1 , Wei‐Ping Deng 1
Affiliation
|
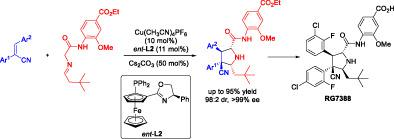
The catalytic asymmetric 1,3‐dipolar [3 + 2] cycloaddition of azomethine ylides with stilbenes has been established, affording structurally diverse pyrrolidines bearing four contiguous stereocenters with stereo‐diversity in generally high yields and good to excellent stereoselectivities (up to 98% yield, 99 : 1 dr, >99% ee). Meanwhile, this strategy allowed the formal synthesis of antitumor drug RG7388 (Phase III clinical study) and its analogues in high efficiency.
中文翻译:

MDM2拮抗剂RG7388的催化对映选择性形式合成及其类似物
已建立了甲亚胺烷基化物与对苯二甲酸酯的催化不对称1,3-偶极[3 + 2]环加成反应,从而提供了结构多样的吡咯烷,带有四个连续的立体中心,立体中心具有高收率,并且具有良好的优异立体选择性(高达98%的收率) ,99:1 dr,> 99%ee)。同时,该策略允许高效合成抗肿瘤药物RG7388(III期临床研究)。
更新日期:2020-04-22
中文翻译:

MDM2拮抗剂RG7388的催化对映选择性形式合成及其类似物
已建立了甲亚胺烷基化物与对苯二甲酸酯的催化不对称1,3-偶极[3 + 2]环加成反应,从而提供了结构多样的吡咯烷,带有四个连续的立体中心,立体中心具有高收率,并且具有良好的优异立体选择性(高达98%的收率) ,99:1 dr,> 99%ee)。同时,该策略允许高效合成抗肿瘤药物RG7388(III期临床研究)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号