Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring Finger Protein 11 acts on ligand-activated EGFR via the direct interaction with the UIM region of ANKRD13 protein family.
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-27 , DOI: 10.1111/febs.15226 Anna Mattioni 1 , Karsten Boldt 2 , Giulio Auciello 3 , Masayuki Komada 4 , Joshua Z Rappoport 5 , Marius Ueffing 2 , Luisa Castagnoli 1 , Gianni Cesareni 1, 6 , Elena Santonico 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-27 , DOI: 10.1111/febs.15226 Anna Mattioni 1 , Karsten Boldt 2 , Giulio Auciello 3 , Masayuki Komada 4 , Joshua Z Rappoport 5 , Marius Ueffing 2 , Luisa Castagnoli 1 , Gianni Cesareni 1, 6 , Elena Santonico 1
Affiliation
|
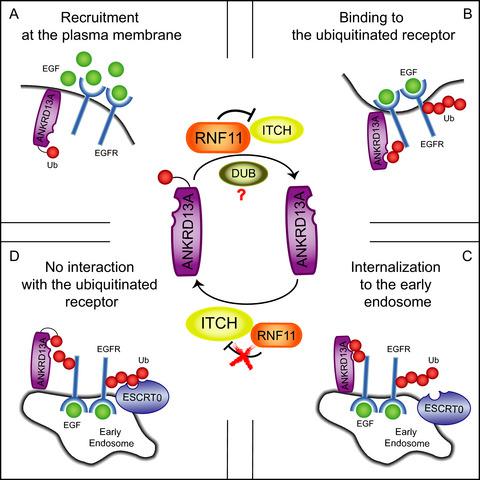
RING finger protein 11 (RNF11) is an evolutionary conserved Really Interesting New Gene E3 ligase that is overexpressed in several human tumours. Although several reports have highlighted its involvement in crucial cellular processes, the mechanistic details underlying its function are still poorly understood. Utilizing stable isotope labelling by amino acids in culture (SILAC)‐based proteomics analysis, we identified 51 proteins that co‐immunoprecipitate with wild‐type RNF11 and/or with its catalytically inactive mutant. We focused our attention on the interaction of RNF11 with Ankyrin repeat domain‐containing protein 13 (ANKRD13)s family. Members of the ANKRD13 family contain ubiquitin‐interacting motifs (UIM) that recognize the Lys‐63‐linked ubiquitin (Ub) chains appended to Epidermal growth factor receptor (EGFR) soon after ligand binding. We show that ANKRD13A, ANKRD13B and ANKRD13D form a complex with RNF11 in vivo and that the UIMs are required for complex formation. However, at odds with the conventional UIM binding mode, Ub modification of RNF11 is not required for the interaction with ANKRD13 proteins. We also show that the interaction between ANKRD13A and RNF11 is modulated by the EGF stimulus and that a complex formed by ANKRD13A, RNF11 and activated EGFR is transiently assembled in the early phases of receptor endocytosis. Moreover, loss of function of the E3 ligases Itchy E3 ubiquitin‐protein ligase (ITCH) or RNF11, respectively, abrogates or increases the ubiquitination of endogenous ANKRD13A, affecting its ability to bind activated EGFR. We propose a model whereby the ANKRD13 proteins act as molecular scaffolds that promote the transient formation of a complex between the activated EGFR and the E3 ligases ITCH and RNF11. By regulating the ubiquitination status of ANKRD13A and consequently its endocytic adaptor function, RNF11 promotes sorting of the activated EGFR for lysosomal degradation.
中文翻译:

无名指蛋白11通过与ANKRD13蛋白家族的UIM区直接相互作用而作用于配体激活的EGFR。
无名指蛋白11(RNF11)是一种进化保守的真正有趣的新基因E3连接酶,在几种人类肿瘤中均过表达。尽管有几篇报道强调了它在关键细胞过程中的作用,但其功能背后的机制细节仍知之甚少。利用基于培养物中氨基酸的稳定同位素标记(SILAC)进行的蛋白质组学分析,我们鉴定了与野生型RNF11和/或其催化失活突变体共免疫沉淀的51种蛋白质。我们将注意力集中在RNF11与锚蛋白重复域含蛋白13(ANKRD13)家族的相互作用上。ANKRD13家族的成员包含泛素相互作用基序(UIM),可识别配体结合后不久与表皮生长因子受体(EGFR)相连的Lys-63连接的泛素(Ub)链。体内而且,UIM是复杂形成所必需的。但是,与常规UIM绑定模式不同,RNF11的Ub修饰对于与ANKRD13蛋白的相互作用不是必需的。我们还表明,ANKRD13A和RNF11之间的相互作用是由EGF刺激调节的,并且由ANKRD13A,RNF11和活化的EGFR形成的复合物在受体内吞作用的早期阶段是瞬时组装的。此外,E3连接酶功能的丧失痒的E3泛素蛋白连接酶(ITCH)或RNF11会消除或增加内源性ANKRD13A的泛素化,从而影响其结合活化的EGFR的能力。我们提出了一种模型,其中ANKRD13蛋白充当分子支架,可促进激活的EGFR与E3连接酶ITCH和RNF11之间复合物的瞬时形成。
更新日期:2020-01-27
中文翻译:

无名指蛋白11通过与ANKRD13蛋白家族的UIM区直接相互作用而作用于配体激活的EGFR。
无名指蛋白11(RNF11)是一种进化保守的真正有趣的新基因E3连接酶,在几种人类肿瘤中均过表达。尽管有几篇报道强调了它在关键细胞过程中的作用,但其功能背后的机制细节仍知之甚少。利用基于培养物中氨基酸的稳定同位素标记(SILAC)进行的蛋白质组学分析,我们鉴定了与野生型RNF11和/或其催化失活突变体共免疫沉淀的51种蛋白质。我们将注意力集中在RNF11与锚蛋白重复域含蛋白13(ANKRD13)家族的相互作用上。ANKRD13家族的成员包含泛素相互作用基序(UIM),可识别配体结合后不久与表皮生长因子受体(EGFR)相连的Lys-63连接的泛素(Ub)链。体内而且,UIM是复杂形成所必需的。但是,与常规UIM绑定模式不同,RNF11的Ub修饰对于与ANKRD13蛋白的相互作用不是必需的。我们还表明,ANKRD13A和RNF11之间的相互作用是由EGF刺激调节的,并且由ANKRD13A,RNF11和活化的EGFR形成的复合物在受体内吞作用的早期阶段是瞬时组装的。此外,E3连接酶功能的丧失痒的E3泛素蛋白连接酶(ITCH)或RNF11会消除或增加内源性ANKRD13A的泛素化,从而影响其结合活化的EGFR的能力。我们提出了一种模型,其中ANKRD13蛋白充当分子支架,可促进激活的EGFR与E3连接酶ITCH和RNF11之间复合物的瞬时形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号