Nature Catalysis ( IF 37.8 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41929-019-0417-1 Zachary J. Garlets , Jacob N. Sanders , Hasnain Malik , Christian Gampe , K. N. Houk , Huw M. L. Davies
|
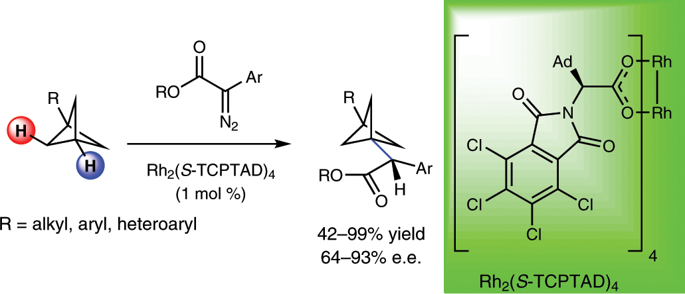
Bicyclo[1.1.1]pentanes (BCPs) are highly strained carbocycles that have fascinated the chemical community for decades because of their unique structure. Despite the immense interest in this scaffold and extensive synthetic efforts, the construction of BCP derivatives still relies substantially on the manipulation of dimethyl bicyclo[1.1.1]pentane-1,3-dicarboxylate. Furthermore, BCPs that contain a proximal stereocentre are underrepresented in the literature and their generation requires stoichiometric chiral auxiliaries. Here we explore enantioselective C–H functionalization of BCPs as a conceptually innovative strategy that provides access to chiral substituted BCPs. For this purpose, enantioselective intermolecular sp3 C–H insertion reactions of donor/acceptor diazo compounds catalysed by the chiral dirhodium complex, Rh2(TCPTAD)4, were employed to forge new C–C bonds at the tertiary position of a variety of BCPs. This work also establishes that highly strained molecules can undergo direct C–H insertion without losing the integrity of their carbocyclic framework.
中文翻译:

双环[1.1.1]戊烷的对映选择性CH官能团
双环[1.1.1]戊烷(BCP)是高度应变的碳环,数十年来因其独特的结构而着迷于化学界。尽管对该支架有极大的兴趣并进行了广泛的合成努力,但BCP衍生物的构建仍主要依赖于对二环双环[1.1.1]戊烷-1,3-二羧酸二甲酯的操作。此外,包含近端立体中心的BCP在文献中的代表性不足,其生成需要化学计量的手性助剂。在这里,我们探讨了BCP的对映选择性C–H功能化,作为一种概念创新策略,可提供手性取代BCP的途径。为此,对映选择性分子间sp 3手性二铑配合物Rh 2(TCPTAD)4催化的供体/受体重氮化合物的C–H插入反应被用来在各种BCP的叔位置建立新的C–C键。这项工作还证明,高应变分子可以直接进行C–H插入而不会丢失其碳环骨架的完整性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号