当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure of the Bcs1 AAA-ATPase suggests an airlock-like translocation mechanism for folded proteins.
Nature Structural & Molecular Biology ( IF 16.8 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41594-019-0364-1 Lukas Kater 1 , Nikola Wagener 2 , Otto Berninghausen 1 , Thomas Becker 1 , Walter Neupert 3 , Roland Beckmann 1
Nature Structural & Molecular Biology ( IF 16.8 ) Pub Date : 2020-01-27 , DOI: 10.1038/s41594-019-0364-1 Lukas Kater 1 , Nikola Wagener 2 , Otto Berninghausen 1 , Thomas Becker 1 , Walter Neupert 3 , Roland Beckmann 1
Affiliation
|
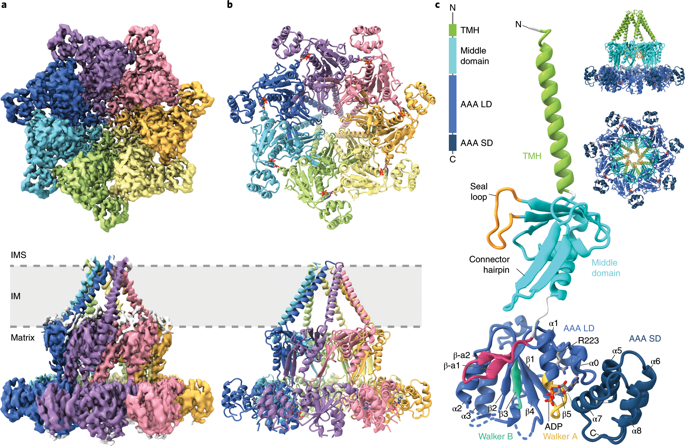
Some proteins require completion of folding before translocation across a membrane into another cellular compartment. Yet the permeability barrier of the membrane should not be compromised and mechanisms have remained mostly elusive. Here, we present the structure of Saccharomyces cerevisiae Bcs1, an AAA-ATPase of the inner mitochondrial membrane. Bcs1 facilitates the translocation of the Rieske protein, Rip1, which requires folding and incorporation of a 2Fe-2S cluster before translocation and subsequent integration into the bc1 complex. Surprisingly, Bcs1 assembles into exclusively heptameric homo-oligomers, with each protomer consisting of an amphipathic transmembrane helix, a middle domain and an ATPase domain. Together they form two aqueous vestibules, the first being accessible from the mitochondrial matrix and the second positioned in the inner membrane, with both separated by the seal-forming middle domain. On the basis of this unique architecture, we propose an airlock-like translocation mechanism for folded Rip1.
中文翻译:

Bcs1 AAA-ATPase的结构表明折叠蛋白的气锁状易位机制。
一些蛋白质需要先完成折叠,然后才能跨膜转运到另一个细胞室内。然而,膜的渗透性屏障不应受到损害,其机理仍然难以捉摸。在这里,我们介绍酿酒酵母Bcs1,线粒体内膜的AAA-ATPase的结构。Bcs1促进了Rieske蛋白Rip1的易位,该蛋白需要折叠并整合2Fe-2S簇,然后才能易位并随后整合到bc1复合物中。出乎意料的是,Bcs1组装成排他性的七聚体均聚物,每个启动子都由两亲性跨膜螺旋,一个中间结构域和一个ATPase结构域组成。它们共同形成两个水性前庭,第一个可以从线粒体基质中获得,第二个可以位于内膜中,两者都由形成密封的中间区域隔开。基于这种独特的体系结构,我们为折叠的Rip1提出了一种类似气闸的易位机制。
更新日期:2020-01-27
中文翻译:

Bcs1 AAA-ATPase的结构表明折叠蛋白的气锁状易位机制。
一些蛋白质需要先完成折叠,然后才能跨膜转运到另一个细胞室内。然而,膜的渗透性屏障不应受到损害,其机理仍然难以捉摸。在这里,我们介绍酿酒酵母Bcs1,线粒体内膜的AAA-ATPase的结构。Bcs1促进了Rieske蛋白Rip1的易位,该蛋白需要折叠并整合2Fe-2S簇,然后才能易位并随后整合到bc1复合物中。出乎意料的是,Bcs1组装成排他性的七聚体均聚物,每个启动子都由两亲性跨膜螺旋,一个中间结构域和一个ATPase结构域组成。它们共同形成两个水性前庭,第一个可以从线粒体基质中获得,第二个可以位于内膜中,两者都由形成密封的中间区域隔开。基于这种独特的体系结构,我们为折叠的Rip1提出了一种类似气闸的易位机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号