当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cleft‐Induced Ditopic Binding of Spherical Halides with a Hexaurea Receptor
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-01-27 , DOI: 10.1002/slct.201903950 Bobby Portis 1 , Ali Mirchi 1 , Mohammad H. Hasan 2 , Maryam Emami Khansari 1 , Corey R. Johnson 1 , Jerzy Leszczynski 1 , Ritesh Tandon 2 , Md. Alamgir Hossain 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-01-27 , DOI: 10.1002/slct.201903950 Bobby Portis 1 , Ali Mirchi 1 , Mohammad H. Hasan 2 , Maryam Emami Khansari 1 , Corey R. Johnson 1 , Jerzy Leszczynski 1 , Ritesh Tandon 2 , Md. Alamgir Hossain 1
Affiliation
|
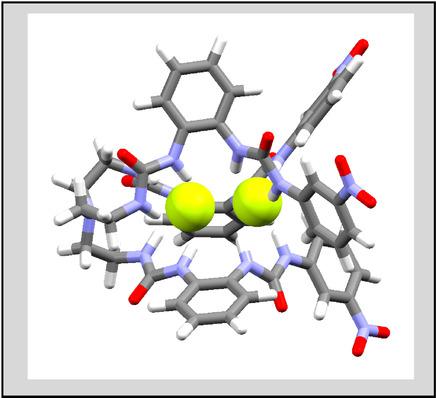
A tripodal‐based hexaurea receptor with two clefts (inner cleft and outer cleft) has been studied for spherical halides by 1H NMR, UV‐Vis titrations, and theoretical calculations using density functional theory (DFT). As demonstrated from experimental and computational results, the receptor exhibits cleft‐induced binding for halides in a 1 : 2 binding mode, showing the binding strength in the order of fluoride > chloride > bromide > iodide. The strongest affinity for fluoride is attributed due to the best fit of two fluoride anions at the two clefts within the host‘s cavity. This is also supported by DFT calculations, suggesting that each anion is stabilized by six NH⋅⋅⋅F bonds ‐ one at the inner cleft and other at the outer cleft. In addition, the synthesized receptor, as examined on HeLa cells, has been found to show good biocompatibility.
中文翻译:

含卤六聚体受体的球形卤化物的裂隙诱导的位点结合
通过1 H NMR,UV-Vis滴定和使用密度泛函理论(DFT)的理论计算,研究了具有两个裂口(内裂和外裂)的基于三脚架的六脲受体。从实验和计算结果证明,该受体以1:2结合模式表现出对卤化物的裂隙诱导结合,显示出结合强度的顺序为氟化物>氯化物>溴化物>碘化物。对氟化物的最强亲和力归因于宿主腔内两个裂缝处的两个氟化物阴离子的最佳配合。DFT计算也证明了这一点,这表明每个阴离子均通过六个NH⋅⋅⋅F键稳定化-一个在内部裂缝处,另一个在内部裂缝处。外裂。另外,如在HeLa细胞上检查的,已发现合成的受体显示出良好的生物相容性。
更新日期:2020-01-27
中文翻译:

含卤六聚体受体的球形卤化物的裂隙诱导的位点结合
通过1 H NMR,UV-Vis滴定和使用密度泛函理论(DFT)的理论计算,研究了具有两个裂口(内裂和外裂)的基于三脚架的六脲受体。从实验和计算结果证明,该受体以1:2结合模式表现出对卤化物的裂隙诱导结合,显示出结合强度的顺序为氟化物>氯化物>溴化物>碘化物。对氟化物的最强亲和力归因于宿主腔内两个裂缝处的两个氟化物阴离子的最佳配合。DFT计算也证明了这一点,这表明每个阴离子均通过六个NH⋅⋅⋅F键稳定化-一个在内部裂缝处,另一个在内部裂缝处。外裂。另外,如在HeLa细胞上检查的,已发现合成的受体显示出良好的生物相容性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号