Progress in Polymer Science ( IF 27.1 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.progpolymsci.2020.101220 Youngwoo Choo , David M. Halat , Irune Villaluenga , Ksenia Timachova , Nitash P. Balsara
|
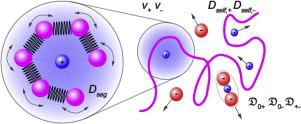
Mixtures of neutral polymers and lithium salts have the potential to serve as electrolytes in next-generation rechargeable Li-ion batteries. The purpose of this review is to expose the delicate interplay between polymer-salt interactions at the segmental level and macroscopic ion transport at the battery level. Since complete characterization of this interplay has only been completed in one system: mixtures of poly(ethylene oxide) and lithium bis(trifluoromethanesulfonyl)imide (PEO/LiTFSI), we focus on data obtained from this system. We begin with a discussion of the activity coefficient, followed by a discussion of six different diffusion coefficients: the Rouse motion of polymer segments is quantified by Dseg, the self-diffusion of cations and anions is quantified by Dself,+ and Dself,−, and the build-up of concentration gradients in electrolytes under an applied potential is quantified by Stefan-Maxwell diffusion coefficients, , , and . The Stefan-Maxwell diffusion coefficients can be used to predict the velocities of the ions at very early times after an electric field is applied across the electrolyte. The surprising result is that is negative in certain concentration windows. A consequence of this finding is that at these concentrations, both cations and anions are predicted to migrate toward the positive electrode at early times. We describe the controversies that surround this result. Knowledge of the Stefan-Maxwell diffusion coefficients enable prediction of the limiting current. We argue that the limiting current is the most important characteristic of an electrolyte. Excellent agreement between theoretical and experimental limiting current is seen in PEO/LiTFSI mixtures. What sequence of monomers that, when polymerized, will lead to the highest limiting current remains an important unanswered question. It is our hope that the approach presented in this review will guide the development of such polymers.
中文翻译:

聚合物电解质中的扩散和迁移
中性聚合物和锂盐的混合物有潜力在下一代可充电锂离子电池中用作电解质。这篇综述的目的是揭示分段水平的聚合物-盐相互作用与电池水平的宏观离子迁移之间的微妙相互作用。由于这种相互作用的完整表征仅在一个系统中完成:聚环氧乙烷和双(三氟甲磺酰基)酰亚胺锂(PEO / LiTFSI)的混合物,因此我们重点研究从该系统获得的数据。我们先讨论活度系数,然后讨论六个不同的扩散系数:聚合物链段的起伏运动用D seg量化,阳离子和阴离子的自扩散用D量化。self,+和D self, -,以及在施加电势下电解质中浓度梯度的建立,通过Stefan-Maxwell扩散系数来量化,, 和 。Stefan-Maxwell扩散系数可用于在电场跨电解质施加后的非常早的时候预测离子的速度。令人惊讶的结果是在某些浓度窗口中为负。这一发现的结果是,在这些浓度下,预计阳离子和阴离子都会在早期向正极迁移。我们描述围绕该结果的争议。了解Stefan-Maxwell扩散系数可以预测极限电流。我们认为极限电流是电解质的最重要特征。在PEO / LiTFSI混合物中可以看到理论和实验极限电流之间的极佳一致性。聚合时将导致最高极限电流的单体顺序是一个重要的未解决问题。我们希望本综述中介绍的方法将指导此类聚合物的开发。


























 京公网安备 11010802027423号
京公网安备 11010802027423号