Environmental Pollution ( IF 8.9 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.envpol.2020.114046 Yao Luo 1 , Jiayu Ding 1 , Ju Hai 1 , Wenfeng Tan 1 , Rong Hao 1 , Guohong Qiu 1
|
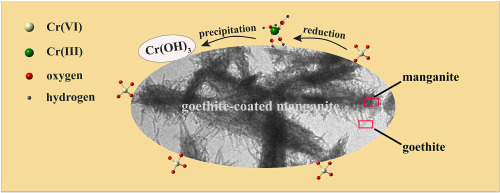
Hexavalent chromium has aroused a series of environmental concerns due to its high mobility and toxicity. Iron and manganese oxides usually coexist in the environments and influence the speciation and geochemical cycling of chromium. However, the interaction mechanism of iron-manganese oxides with dissolved Cr(VI) remains largely unknown. In this work, the interaction processes of dissolved Cr(VI) and manganite in the presence of goethite coating were investigated, and the effects of pH (2.0–9.0) and iron oxide content were also studied. Manganite-goethite composites were formed with uniform micromorphologies in the system of manganite and Fe(II). In the reaction system of single manganite and Cr(VI), manganite could only adsorb but not reduce Cr(VI), with the adsorption amount decreasing at higher pHs. In the reaction system of manganite-goethite composites and Cr(VI), adsorbed Cr(VI) was reduced to Cr(III) by Fe(II) on composites surface. The generated Cr(III) was then retained as Cr(OH)3 on the mineral surface. Goethite coating suppressed the re-oxidation of newly formed Cr(III) by manganite. The amounts of adsorbed Cr(VI) and generated Cr(III) increased with increasing iron oxide content, and increased first and then decreased with increasing pH. The Cr(III) formation and Cr(VI) adsorption amount reached the maximum at pH 5.0–6.0. The present work highlights the transformation and retention of Cr(VI) by iron-manganese oxides and provides potential implications for the use of such oxides in the remediation of Cr(VI) polluted waters and soils.
中文翻译:

针铁矿涂层存在下Cr(VI)与锰的溶解机理
六价铬由于其高迁移率和毒性而引起了一系列环境问题。铁和锰的氧化物通常在环境中共存,并影响铬的形态和地球化学循环。但是,铁锰氧化物与溶解的Cr(VI)的相互作用机理仍然未知。在这项工作中,研究了在针铁矿涂层存在下溶解的Cr(VI)和锰矿的相互作用过程,并研究了pH(2.0-9.0)和氧化铁含量的影响。在锰铁和铁(II)的体系中形成了具有均匀微观形貌的锰铁-针铁矿复合材料。在单一锰矿与Cr(VI)的反应体系中,锰矿只能吸附而不还原Cr(VI),在较高的pH值下吸附量会减少。在锰铁-针铁矿复合材料与Cr(VI)的反应体系中,复合材料表面上的Fe(II)将吸附的Cr(VI)还原为Cr(III)。然后将生成的Cr(III)保留为Cr(OH)3在矿物表面。针铁矿涂层抑制了锰形成的新形成的Cr(III)的再氧化。Cr(VI)和生成的Cr(III)的吸附量随氧化铁含量的增加而增加,并随pH值的增加先增加后减少。在pH 5.0-6.0时,Cr(III)的形成和Cr(VI)的吸附量达到最大值。本工作着重说明了铁锰氧化物对Cr(VI)的转化和保留,并为在修复Cr(VI)污染的水和土壤中使用此类氧化物提供了潜在的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号