当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Annulation of Hydrazones and Alkynes via Rhodium(III)-Catalyzed Dual C-H Activation: Synthesis of Pyrrolopyridazines and Azolopyridazines.
Organic Letters ( IF 5.2 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.orglett.0c00186 Andrew D Streit 1 , Adam J Zoll 1 , Gia L Hoang 1 , Jonathan A Ellman 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.orglett.0c00186 Andrew D Streit 1 , Adam J Zoll 1 , Gia L Hoang 1 , Jonathan A Ellman 1
Affiliation
|
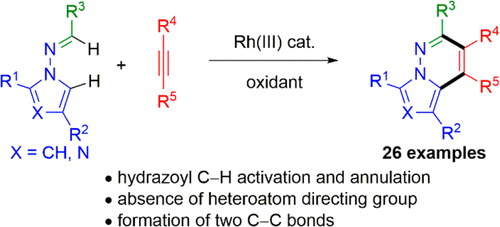
Hydrazones readily synthesized from N-aminopyrroles or N-aminoazoles and aldehydes undergo Rh(III)-catalyzed dual C-H activation and coupling with aryl- and alkyl-substituted alkynes to give pyrrolopyridazines or azolopyridazines, respectively. This transformation represents a rare example of hydrazoyl C-H activation and proceeds without heteroatom functionality to direct C-H activation. Hydrazones derived from aromatic, alkenyl, and aliphatic aldehydes were effective inputs, and tethering the alkyne to the hydrazone enabled annulations to more complex, tricyclic products.
中文翻译:

通过铑(III)催化的双CH活化对dra和炔烃的环化反应:吡咯并哒嗪和氮杂哒嗪的合成。
由N-氨基吡咯或N-氨基唑和醛容易合成的经过Rh(III)催化的双CH活化,并与芳基和烷基取代的炔烃偶联,分别得到吡咯并哒嗪或氮杂哒嗪。该转化代表了酰肼基CH活化的罕见例子,并且在没有杂原子官能团的情况下继续进行以指导CH活化。衍生自芳族,烯基和脂族醛的是有效的输入,并且将炔烃与to拴系可以使环化成更复杂的三环产物。
更新日期:2020-01-26
中文翻译:

通过铑(III)催化的双CH活化对dra和炔烃的环化反应:吡咯并哒嗪和氮杂哒嗪的合成。
由N-氨基吡咯或N-氨基唑和醛容易合成的经过Rh(III)催化的双CH活化,并与芳基和烷基取代的炔烃偶联,分别得到吡咯并哒嗪或氮杂哒嗪。该转化代表了酰肼基CH活化的罕见例子,并且在没有杂原子官能团的情况下继续进行以指导CH活化。衍生自芳族,烯基和脂族醛的是有效的输入,并且将炔烃与to拴系可以使环化成更复杂的三环产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号