当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How to bend a cumulene.
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-01-25 , DOI: 10.1002/chem.202000025 José Enrique Barquera-Lozada 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-01-25 , DOI: 10.1002/chem.202000025 José Enrique Barquera-Lozada 1
Affiliation
|
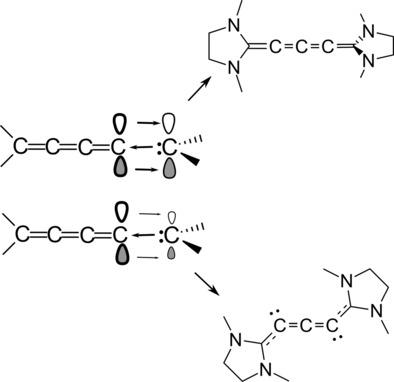
Allenes (carbodicarbenes) and [3]cumulenes are linear carbon chains, which can be bent when the terminal group has a strong carbine nature. This bending can be quite pronounced in allenes but not in [3]cumulenes. We analyse how N-heterocyclic or cyclic (alkyl)-(amino) carbenes (NHC and CAAC, respectively) terminal groups can modify the linear structure of [n]cumulenes. A low π-acidity of the terminal carbene affects the linearity of [2n]cumulenes. We found, indeed, that the NHC [4]cumulene is extremely bent, contrary to classical [4]cumulenes. The predicted NHC [4]cumulene or tricarbodicarbene has two lone pairs and the π-electrons are delocalized through the whole molecule. More significantly, the DFT calculations show that this bent [4]cumulene is very stable, considerably more than its corresponding [3]cumulene that has been elusive to synthesize. Remarkably, the calculations show that all the NHC [2n]cumulenes are more than 25 kcal/mol more stable than [2n-1]cumulenes.
中文翻译:

如何弯曲异丙苯。
丙二烯(碳化二碳烯)和[3]枯烯类是线性碳链,当末端基团具有很强的卡宾性质时,它们可以弯曲。这种弯曲在艾伦烯中非常明显,但在[3]枯草烯中却不明显。我们分析了N-杂环或环状(烷基)-(氨基)碳烯(分别为NHC和CAAC)末端基团可如何修饰[n] cumulenes的线性结构。末端卡宾的低π酸度会影响[2n]枯烯的线性。实际上,我们发现,NHC [4]异丙苯与常规的[4]枯烯烯相反,它极易弯曲。预测的NHC [4]异丙苯或三碳二碳烯具有两个孤对,并且π电子在整个分子中都离域。更重要的是,DFT计算表明,这种弯曲的[4]异丙苯非常稳定,比其难以合成的相应[3]异丙苯多得多。值得注意的是,计算表明,所有的NHC [2n]枯草酮比[2n-1]枯草素的稳定性要高25 kcal / mol。
更新日期:2020-03-19
中文翻译:

如何弯曲异丙苯。
丙二烯(碳化二碳烯)和[3]枯烯类是线性碳链,当末端基团具有很强的卡宾性质时,它们可以弯曲。这种弯曲在艾伦烯中非常明显,但在[3]枯草烯中却不明显。我们分析了N-杂环或环状(烷基)-(氨基)碳烯(分别为NHC和CAAC)末端基团可如何修饰[n] cumulenes的线性结构。末端卡宾的低π酸度会影响[2n]枯烯的线性。实际上,我们发现,NHC [4]异丙苯与常规的[4]枯烯烯相反,它极易弯曲。预测的NHC [4]异丙苯或三碳二碳烯具有两个孤对,并且π电子在整个分子中都离域。更重要的是,DFT计算表明,这种弯曲的[4]异丙苯非常稳定,比其难以合成的相应[3]异丙苯多得多。值得注意的是,计算表明,所有的NHC [2n]枯草酮比[2n-1]枯草素的稳定性要高25 kcal / mol。


























 京公网安备 11010802027423号
京公网安备 11010802027423号