当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Manipulating the Geometric and Electronic Structures of Manganese Nitrides for Ammonia Synthesis
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-11 , DOI: 10.1002/cctc.201902383 Nannan Shan 1 , Chaoran Huang 1 , Robert T. Lee 1 , Narges Manavi 1 , Lianbin Xu 2 , Viktor Chikan 3 , Peter Heinz Pfromm 1, 4 , Bin Liu 1
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-11 , DOI: 10.1002/cctc.201902383 Nannan Shan 1 , Chaoran Huang 1 , Robert T. Lee 1 , Narges Manavi 1 , Lianbin Xu 2 , Viktor Chikan 3 , Peter Heinz Pfromm 1, 4 , Bin Liu 1
Affiliation
|
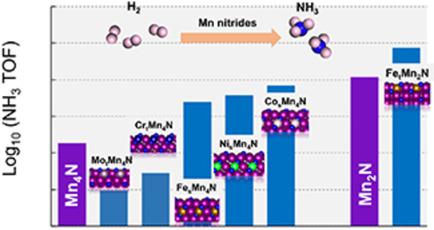
Manganese (Mn) nitrides are important nitrogen (N) carriers for small‐scale intermittent ammonia (NH3) synthesis. However, only 3∼8 % of lattice N are converted into NH3. In this study, the geometric and electronic structures of well‐defined Mn4N and Mn2N lattices were altered using transition metal heteroatoms (Cr, Fe, Co, Ni, Mo) to understand the driving force behind lattice N diffusion and extraction. Density Functional Theory (DFT) revealed that the binding of early hydrogenation product (NH) follows a linear relationship with lattice N over a wide range of close‐packed surfaces. But, the binding of NH2 and NH3 are more sensitive to the geometric and electronic structures. Further, the chemical bonding can be quantitatively characterized with the covalency derived from Crystal Orbital Hamiltonian Population (COHP). In the Eley Rideal‐Mars van Krevelan pathway, the overall NH3 formation free energy ( ) and lattice N diffusion barrier (
) and lattice N diffusion barrier ( ) are the respective thermodynamic and kinetic determining factors. Aided by a rate‐determining step (RDS) model, Mn4N modified with Fe, Co, Ni single‐atom dopants all show enhanced rates for NH3 formation.
) are the respective thermodynamic and kinetic determining factors. Aided by a rate‐determining step (RDS) model, Mn4N modified with Fe, Co, Ni single‐atom dopants all show enhanced rates for NH3 formation.
中文翻译:

操纵合成氮化锰的几何结构和电子结构
锰(Mn)氮化物是小规模间歇性氨(NH 3)合成的重要氮(N)载体。但是,只有3〜8%的晶格N被转化为NH 3。在这项研究中,使用过渡金属杂原子(Cr,Fe,Co,Ni,Mo)改变了明确定义的Mn 4 N和Mn 2 N晶格的几何和电子结构,以了解晶格N扩散和提取的驱动力。密度泛函理论(DFT)显示,早期氢化产物(NH)的结合在大面积密排表面上与晶格N呈线性关系。但是,NH 2和NH 3的结合对几何和电子结构更敏感。此外,化学键合可通过衍生自晶体轨道哈密顿量(COHP)的共价性进行定量表征。在Eley Rideal-Mars van Krevelan路径中,总的NH 3形成自由能( )和晶格N扩散势垒(
)和晶格N扩散势垒( )是各自的热力学和动力学决定因素。在速率决定步骤(RDS)模型的辅助下,用Fe,Co,Ni单原子掺杂剂改性的Mn 4 N均显示出更高的NH 3形成速率。
)是各自的热力学和动力学决定因素。在速率决定步骤(RDS)模型的辅助下,用Fe,Co,Ni单原子掺杂剂改性的Mn 4 N均显示出更高的NH 3形成速率。
更新日期:2020-04-22
 ) and lattice N diffusion barrier (
) and lattice N diffusion barrier ( ) are the respective thermodynamic and kinetic determining factors. Aided by a rate‐determining step (RDS) model, Mn4N modified with Fe, Co, Ni single‐atom dopants all show enhanced rates for NH3 formation.
) are the respective thermodynamic and kinetic determining factors. Aided by a rate‐determining step (RDS) model, Mn4N modified with Fe, Co, Ni single‐atom dopants all show enhanced rates for NH3 formation.
中文翻译:

操纵合成氮化锰的几何结构和电子结构
锰(Mn)氮化物是小规模间歇性氨(NH 3)合成的重要氮(N)载体。但是,只有3〜8%的晶格N被转化为NH 3。在这项研究中,使用过渡金属杂原子(Cr,Fe,Co,Ni,Mo)改变了明确定义的Mn 4 N和Mn 2 N晶格的几何和电子结构,以了解晶格N扩散和提取的驱动力。密度泛函理论(DFT)显示,早期氢化产物(NH)的结合在大面积密排表面上与晶格N呈线性关系。但是,NH 2和NH 3的结合对几何和电子结构更敏感。此外,化学键合可通过衍生自晶体轨道哈密顿量(COHP)的共价性进行定量表征。在Eley Rideal-Mars van Krevelan路径中,总的NH 3形成自由能(
 )和晶格N扩散势垒(
)和晶格N扩散势垒( )是各自的热力学和动力学决定因素。在速率决定步骤(RDS)模型的辅助下,用Fe,Co,Ni单原子掺杂剂改性的Mn 4 N均显示出更高的NH 3形成速率。
)是各自的热力学和动力学决定因素。在速率决定步骤(RDS)模型的辅助下,用Fe,Co,Ni单原子掺杂剂改性的Mn 4 N均显示出更高的NH 3形成速率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号