当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Predictive Model for Delivery Efficiency: Erythrocyte Membrane-Camouflaged Magnetofluorescent Nanocarriers Study.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.molpharmaceut.9b01094 Ailton A Sousa-Junior 1 , Sebastião A Mendanha 1 , Marcus S Carrião 1 , Gustavo Capistrano 1 , André G Próspero 2 , Guilherme A Soares 2 , Emílio R Cintra 3 , Sônia F O Santos 4 , Nicholas Zufelato 1 , Antônio Alonso 1 , Eliana M Lima 3 , José Ricardo A Miranda 2 , Elisângela de P Silveira-Lacerda 4 , Cléver G Cardoso 4 , Andris F Bakuzis 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-02-06 , DOI: 10.1021/acs.molpharmaceut.9b01094 Ailton A Sousa-Junior 1 , Sebastião A Mendanha 1 , Marcus S Carrião 1 , Gustavo Capistrano 1 , André G Próspero 2 , Guilherme A Soares 2 , Emílio R Cintra 3 , Sônia F O Santos 4 , Nicholas Zufelato 1 , Antônio Alonso 1 , Eliana M Lima 3 , José Ricardo A Miranda 2 , Elisângela de P Silveira-Lacerda 4 , Cléver G Cardoso 4 , Andris F Bakuzis 1
Affiliation
|
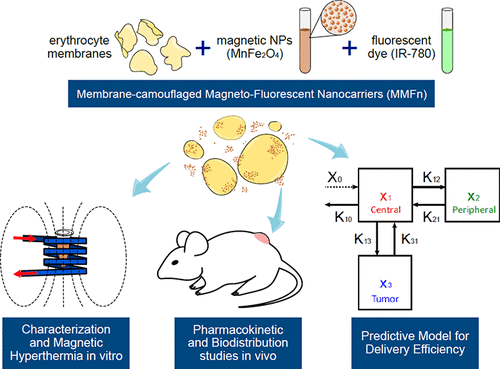
Delivery efficiencies of theranostic nanoparticles (NPs) based on passive tumor targeting strongly depend either on their blood circulation time or on appropriate modulations of the tumor microenvironment. Therefore, predicting the NP delivery efficiency before and after a tumor microenvironment modulation is highly desirable. Here, we present a new erythrocyte membrane-camouflaged magnetofluorescent nanocarrier (MMFn) with long blood circulation time (92 h) and high delivery efficiency (10% ID for Ehrlich murine tumor model). MMFns owe their magnetic and fluorescent properties to the incorporation of manganese ferrite nanoparticles (MnFe2O4 NPs) and IR-780 (a lipophilic indocyanine fluorescent dye), respectively, to their erythrocyte membrane-derived camouflage. MMFn composition, morphology, and size, as well as optical absorption, zeta potential, and fluorescent, magnetic, and magnetothermal properties, are thoroughly examined in vitro. We then present an analytical pharmacokinetic (PK) model capable of predicting the delivery efficiency (DE) and the time of peak tumor uptake (tmax), as well as changes in DE and tmax due to modulations of the tumor microenvironment, for potentially any nanocarrier. Experimental PK data sets (blood and tumor amounts of MMFns) are simultaneously fit to the model equations using the PK modeling software Monolix. We then validate our model analytical solutions with the numerical solutions provided by Monolix. We also demonstrate how our a priori nonmechanistic model for passive targeting relates to a previously reported mechanistic model for active targeting. All in vivo PK studies, as well as in vivo and ex vivo biodistribution studies, were conducted using two noninvasive techniques, namely, fluorescence molecular tomography (FMT) and alternating current biosusceptometry (ACB). Finally, histopathology corroborates our PK and biodistribution results.
中文翻译:

传递效率的预测模型:红细胞膜伪装的磁荧光纳米载体研究。
基于被动性肿瘤靶向的治疗性纳米颗粒(NP)的递送效率在很大程度上取决于其血液循环时间或对肿瘤微环境的适当调节。因此,非常需要预测在肿瘤微环境调节之前和之后的NP递送效率。在这里,我们提出了一种新的红细胞膜伪装的磁荧光纳米载体(MMFn),具有长的血液循环时间(92 h)和高的递送效率(Ehrlich鼠肿瘤模型的ID为10%)。MMFns的磁性和荧光特性归因于锰铁氧体纳米粒子(MnFe2O4 NPs)和IR-780(亲脂性吲哚菁荧光染料)分别掺入了其红细胞膜来源的伪装。MMFn的组成,形态和大小,以及光吸收,ζ电势,荧光,磁性和磁热性质已在体外进行了彻底检查。然后,我们提出了一种解析药代动力学(PK)模型,该模型能够预测潜在的任何纳米载体的递送效率(DE)和最大肿瘤吸收时间(tmax)以及由于肿瘤微环境的调节而引起的DE和tmax的变化。 。使用PK建模软件Monolix同时将实验PK数据集(MMFns的血液和肿瘤量)与模型方程拟合。然后,我们使用Monolix提供的数值解决方案验证模型分析解决方案。我们还演示了用于被动目标的先验非机械模型如何与先前报告的用于主动目标的机械模型有关。所有体内PK研究以及体内和离体生物分布研究,我们使用两种非侵入性技术进行了研究,即荧光分子断层扫描(FMT)和交流电生物电法(ACB)。最后,组织病理学证实了我们的PK和生物分布结果。
更新日期:2020-02-06
中文翻译:

传递效率的预测模型:红细胞膜伪装的磁荧光纳米载体研究。
基于被动性肿瘤靶向的治疗性纳米颗粒(NP)的递送效率在很大程度上取决于其血液循环时间或对肿瘤微环境的适当调节。因此,非常需要预测在肿瘤微环境调节之前和之后的NP递送效率。在这里,我们提出了一种新的红细胞膜伪装的磁荧光纳米载体(MMFn),具有长的血液循环时间(92 h)和高的递送效率(Ehrlich鼠肿瘤模型的ID为10%)。MMFns的磁性和荧光特性归因于锰铁氧体纳米粒子(MnFe2O4 NPs)和IR-780(亲脂性吲哚菁荧光染料)分别掺入了其红细胞膜来源的伪装。MMFn的组成,形态和大小,以及光吸收,ζ电势,荧光,磁性和磁热性质已在体外进行了彻底检查。然后,我们提出了一种解析药代动力学(PK)模型,该模型能够预测潜在的任何纳米载体的递送效率(DE)和最大肿瘤吸收时间(tmax)以及由于肿瘤微环境的调节而引起的DE和tmax的变化。 。使用PK建模软件Monolix同时将实验PK数据集(MMFns的血液和肿瘤量)与模型方程拟合。然后,我们使用Monolix提供的数值解决方案验证模型分析解决方案。我们还演示了用于被动目标的先验非机械模型如何与先前报告的用于主动目标的机械模型有关。所有体内PK研究以及体内和离体生物分布研究,我们使用两种非侵入性技术进行了研究,即荧光分子断层扫描(FMT)和交流电生物电法(ACB)。最后,组织病理学证实了我们的PK和生物分布结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号