当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In-silico epitope identification and design of Uricase mutein with reduced immunogenicity
Process Biochemistry ( IF 4.4 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.procbio.2020.01.022 Anand Kumar Nelapati , Bratin Kumar Das , Jagadeesh Babu Ponnan Ettiyappan , Debashree Chakraborty
Process Biochemistry ( IF 4.4 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.procbio.2020.01.022 Anand Kumar Nelapati , Bratin Kumar Das , Jagadeesh Babu Ponnan Ettiyappan , Debashree Chakraborty
|
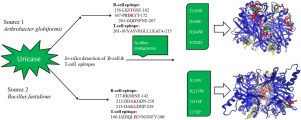
Abstract The clinical utilization of Uricase against gout is limited due to the immunogenicity. In the present article, we identified the antigenic determinants of Uricase and reduced their immunogenicity via in-silico mutagenesis. Multiple sequence alignment and motif analysis were carried out to identify the conserved residues in evolutionary process. Emini surface accessibility, Parker hydrophilicity, and Karplus & Schulz flexibility methods were employed to predict the linear B-cell epitopes of both Ag-Uricase and Bf-Uricase. Deimmunization approach identified T-cell epitopes and the hot spot residues. Reduced antigenic probability was obtained in case of T159W, D169C, N264W and Y203D mutations for Ag-Uricase, while S139 V, K215W, G216 F and I172 P mutations for Bf-Uricase. The binding affinity values of uric acid towards the catalytic pocket of Ag-Uricase and Bf-Uricase models were found to be -48.71 kcal/mol and -40.93 kcal/mol, respectively. This energy is further stabilized in the mutant model by -6.36 kcal/mol and -1.45 kcal/mol for Ag-Uricase and Bf-Uricase, respectively. About 100 ns molecular dynamics simulation was performed to evaluate the conformational stability of both native and mutated Uricase. Insights obtained from this study provide guidelines for experimental design of Uricase muteins with reduced antigenicity.
中文翻译:

具有降低免疫原性的尿酸酶突变蛋白的计算机表位鉴定和设计
摘要 由于免疫原性,尿酸酶治疗痛风的临床应用受到限制。在本文中,我们鉴定了尿酸酶的抗原决定簇,并通过计算机内诱变降低了它们的免疫原性。进行多序列比对和基序分析以鉴定进化过程中的保守残基。Emini 表面可及性、Parker 亲水性和 Karplus & Schulz 灵活性方法被用来预测 Ag-Uricase 和 Bf-Uricase 的线性 B 细胞表位。去免疫方法鉴定了 T 细胞表位和热点残基。在 Ag-尿酸酶的 T159W、D169C、N264W 和 Y203D 突变的情况下,获得降低的抗原概率,而 Bf-尿酸酶的 S139 V、K215W、G216 F 和 I172 P 突变。发现尿酸对 Ag-Uricase 和 Bf-Uricase 模型的催化口袋的结合亲和力值分别为 -48.71 kcal/mol 和 -40.93 kcal/mol。对于 Ag-尿酸酶和 Bf-尿酸酶,该能量在突变模型中进一步稳定为 -6.36 kcal/mol 和 -1.45 kcal/mol。进行了大约 100 ns 的分子动力学模拟以评估天然和突变的尿酸酶的构象稳定性。从这项研究中获得的见解为具有降低抗原性的尿酸酶突变蛋白的实验设计提供了指导。进行了大约 100 ns 的分子动力学模拟以评估天然和突变的尿酸酶的构象稳定性。从这项研究中获得的见解为具有降低抗原性的尿酸酶突变蛋白的实验设计提供了指导。进行了大约 100 ns 的分子动力学模拟以评估天然和突变的尿酸酶的构象稳定性。从这项研究中获得的见解为具有降低抗原性的尿酸酶突变蛋白的实验设计提供了指导。
更新日期:2020-05-01
中文翻译:

具有降低免疫原性的尿酸酶突变蛋白的计算机表位鉴定和设计
摘要 由于免疫原性,尿酸酶治疗痛风的临床应用受到限制。在本文中,我们鉴定了尿酸酶的抗原决定簇,并通过计算机内诱变降低了它们的免疫原性。进行多序列比对和基序分析以鉴定进化过程中的保守残基。Emini 表面可及性、Parker 亲水性和 Karplus & Schulz 灵活性方法被用来预测 Ag-Uricase 和 Bf-Uricase 的线性 B 细胞表位。去免疫方法鉴定了 T 细胞表位和热点残基。在 Ag-尿酸酶的 T159W、D169C、N264W 和 Y203D 突变的情况下,获得降低的抗原概率,而 Bf-尿酸酶的 S139 V、K215W、G216 F 和 I172 P 突变。发现尿酸对 Ag-Uricase 和 Bf-Uricase 模型的催化口袋的结合亲和力值分别为 -48.71 kcal/mol 和 -40.93 kcal/mol。对于 Ag-尿酸酶和 Bf-尿酸酶,该能量在突变模型中进一步稳定为 -6.36 kcal/mol 和 -1.45 kcal/mol。进行了大约 100 ns 的分子动力学模拟以评估天然和突变的尿酸酶的构象稳定性。从这项研究中获得的见解为具有降低抗原性的尿酸酶突变蛋白的实验设计提供了指导。进行了大约 100 ns 的分子动力学模拟以评估天然和突变的尿酸酶的构象稳定性。从这项研究中获得的见解为具有降低抗原性的尿酸酶突变蛋白的实验设计提供了指导。进行了大约 100 ns 的分子动力学模拟以评估天然和突变的尿酸酶的构象稳定性。从这项研究中获得的见解为具有降低抗原性的尿酸酶突变蛋白的实验设计提供了指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号