Electrochimica Acta ( IF 6.6 ) Pub Date : 2020-01-26 , DOI: 10.1016/j.electacta.2020.135785 Q. Abbas , H. Fitzek , V. Pavlenko , B. Gollas
|
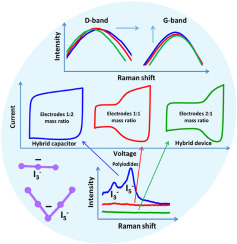
Considering the cost-effectiveness, safety, and environmental friendliness for energy storage and delivery at high rates, hybrid electrochemical capacitors (ECs) in aqueous electrolytes containing redox-active species are attractive alternatives to expensive organic electrolyte based electric double-layer capacitors (EDLCs). Here, the influence of electrode mass-balancing on the equilibrium potential of hybrid cells in aqueous sodium nitrate + sodium iodide (5 mol L−1 NaNO3 + 0.5 mol L−1 NaI) has been investigated. The shift of equilibrium potential determines, whether the positive electrode behaves fully battery-like (charge/discharge strictly in the iodide redox potential range) or shows a mixed battery-like and EDL capacitive behavior. With an appropriate mass-balancing of the positive and negative electrodes (mass ratio = 1:2), the equilibrium potential shows a negligible shift during galvanostatic charge/discharge cycles at 0.5 A g−1, which results in full battery-like behavior of the positive electrode. Consequently, the hybrid cell exhibits stable electrochemical performance. By contrast, an equal or higher mass of the positive compared to the negative electrode, leads to a shift of the equilibrium potential resulting in two different charge storage mechanisms at the positive electrode. As a result, the overall performance of the hybrid cell deteriorates. We show by thermogravimetric analysis and Raman spectroscopy that the formation of polyiodides (I3− and I5−) is controlled by the oxidation of iodide (I−) anions to molecular iodine in nanoporous carbon based positive electrode, and that more polyiodides are produced, if the positive electrode operates strictly within the iodide/iodine redox potential range.
中文翻译:

面向基于碘化物的水性氧化还原电解质中的优化混合电化学电容器:通过电极质量平衡平衡电位的移动
考虑到能量存储的高成本效益,安全性和环境友好性,含有氧化还原活性物质的水性电解质中的混合电化学电容器(EC)是昂贵的基于有机电解质的双电层电容器(EDLC)的有吸引力的替代品。在此,电极质量平衡对硝酸钠水溶液+碘化钠(5 mol L -1 NaNO 3 + 0.5 mol L -1中的杂化电池平衡电位的影响)NaI)已被调查。平衡电位的变化决定了正极是完全像电池一样(严格在碘化物氧化还原电位范围内进行充电/放电),还是表现出混合的电池样和EDL电容特性。在正极和负极的质量平衡适当(质量比= 1:2)的情况下,在恒电流充/放电循环中,在0.5 A g -1时,平衡电势的变化可忽略不计,导致正极具有完全的电池状行为。因此,混合电池表现出稳定的电化学性能。相反,与负极相比,正极的质量相等或更高会导致平衡电位的移动,从而在正极产生两种不同的电荷存储机制。结果,混合电池的整体性能下降。我们通过热重分析和拉曼光谱表明,聚碘(I形成3 -和我5 - )通过碘(I的氧化控制-)在纳米多孔碳基正极中与分子碘形成阴离子,如果正极严格在碘/碘氧化还原电势范围内运行,则会产生更多的聚碘。


























 京公网安备 11010802027423号
京公网安备 11010802027423号