当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydroconversion of 5‐Hydroxymethylfurfural to 2,5‐Dimethylfuran and 2,5‐Dimethyltetrahydrofuran over Non‐promoted Ni/SBA‐15
ChemCatChem ( IF 4.5 ) Pub Date : 2020-02-21 , DOI: 10.1002/cctc.201902028 Shuo Chen 1 , Carmen Ciotonea 1 , Karine Vigier 2 , François Jérôme 2 , Robert Wojcieszak 3 , Franck Dumeignil 1 , Eric Marceau 1 , Sébastien Royer 4
ChemCatChem ( IF 4.5 ) Pub Date : 2020-02-21 , DOI: 10.1002/cctc.201902028 Shuo Chen 1 , Carmen Ciotonea 1 , Karine Vigier 2 , François Jérôme 2 , Robert Wojcieszak 3 , Franck Dumeignil 1 , Eric Marceau 1 , Sébastien Royer 4
Affiliation
|
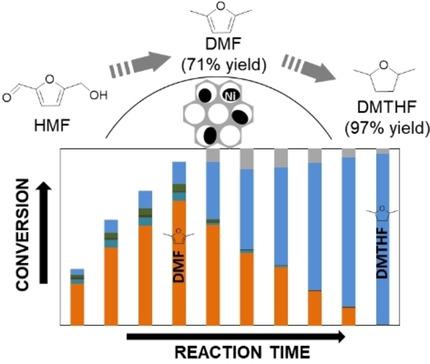
The selective hydroconversion of 5‐hydroxymethylfurfural (HMF) to biofuels is currently highly sought‐for. While the literature has demonstrated that this reaction is possible on promoted Ni catalysts, we show here that a monometallic, non‐promoted Ni/SBA‐15 catalyst, prepared by incipient wetness impregnation, can convert HMF to 2,5‐dimethylfuran (DMF) and to 2,5‐dimethyltetrahydrofuran (DMTHF) at 180 °C, in a consecutive way. Through a control over reaction time, high yields to DMF (71 %, at conversion of 93 %) or DMTHF (97 %, at conversion of 100 %) can be achieved. Kinetic modelling suggests a preferential route to DMF via 5‐methylfurfural (MFFR) as intermediate, though the route via 2,5‐bis(hydroxylmethyl)furan (BHMF) is also present. The favored route in the experimental conditions involves the hydrogenolysis of the hydroxyl group of HMF as first step, followed by the hydrogenation of the aldehyde function, to methylfurfuryl alcohol (MFOL). It is suggested a higher reaction rate of hydrogenation or hydrogenolysis of the side group is linked to the presence of a methyl group in the molecule. No hydrogenation of the furan ring is detected on the intermediates.
中文翻译:

在未促进的Ni / SBA-15上将5-羟基甲基糠醛加氢转化为2,5-二甲基呋喃和2,5-二甲基四氢呋喃
目前,人们强烈要求将5-羟甲基糠醛(HMF)选择性加氢转化为生物燃料。尽管文献已经表明该反应在促进型Ni催化剂上是可能的,但我们在这里表明通过初期湿润浸渍制备的单金属,非促进型Ni / SBA-15催化剂可以将HMF转化为2,5-二甲基呋喃(DMF)并以连续方式在180°C下加入2,5-二甲基四氢呋喃(DMTHF)。通过控制反应时间,可以实现高产率的DMF(71%,转化率93%)或DMTHF(97%,转化率100%)。动力学模型表明,通过5-甲基糠醛(MFFR)作为中间体的DMF优先路线,尽管也存在通过2,5-双(羟甲基)呋喃(BHMF)的路线。在实验条件下优选的途径包括第一步是将HMF的羟基氢解,然后将醛官能团氢化为甲基糠醇(MFOL)。建议侧基的氢化或氢解的更高反应速率与分子中甲基的存在有关。在中间体上未检测到呋喃环的氢化。
更新日期:2020-02-21
中文翻译:

在未促进的Ni / SBA-15上将5-羟基甲基糠醛加氢转化为2,5-二甲基呋喃和2,5-二甲基四氢呋喃
目前,人们强烈要求将5-羟甲基糠醛(HMF)选择性加氢转化为生物燃料。尽管文献已经表明该反应在促进型Ni催化剂上是可能的,但我们在这里表明通过初期湿润浸渍制备的单金属,非促进型Ni / SBA-15催化剂可以将HMF转化为2,5-二甲基呋喃(DMF)并以连续方式在180°C下加入2,5-二甲基四氢呋喃(DMTHF)。通过控制反应时间,可以实现高产率的DMF(71%,转化率93%)或DMTHF(97%,转化率100%)。动力学模型表明,通过5-甲基糠醛(MFFR)作为中间体的DMF优先路线,尽管也存在通过2,5-双(羟甲基)呋喃(BHMF)的路线。在实验条件下优选的途径包括第一步是将HMF的羟基氢解,然后将醛官能团氢化为甲基糠醇(MFOL)。建议侧基的氢化或氢解的更高反应速率与分子中甲基的存在有关。在中间体上未检测到呋喃环的氢化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号