Progress in Retinal and Eye Research ( IF 17.8 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.preteyeres.2020.100840 Eun Jung Lee 1 , Jong Chul Han 1 , Do Young Park 1 , Changwon Kee 1
|
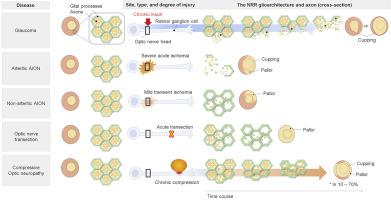
Neuroretinal rim thinning (NRR) is a characteristic glaucomatous optic disc change. However, the precise mechanism of the rim thinning has not been completely elucidated. This review focuses on the structural role of the glioarchitecture in the formation of the glaucomatous NRR thinning. The NRR is a glia-framed structure, with honeycomb geometry and mechanically reinforced astrocyte processes along the transverse plane. When neural damage selectively involves the neuron and spares the glia, the gross structure of the tissue is preserved. The disorganization and loss of the glioarchitecture are the two hallmarks of optic nerve head (ONH) remodeling in glaucoma that leads to the thinning of NRR tissue upon axonal loss. This is in contrast to most non-glaucomatous optic neuropathies with optic disc pallor where hypertrophy of the glioarchitecture is associated with the seemingly absent optic disc cupping. Arteritic anterior ischemic optic neuropathy is an exception where pan-necrosis of ONH tissue leads to NRR thinning. Milder ischemia indicates selective neuronal loss that spares glia in non-arteritic anterior ischemic optic neuropathy. The biological reason is the heterogeneous glial response determined by the site, type, and severity of the injury. The neuroglial interpretation explains how the cellular changes underlie the clinical findings. Updated understandings on glial responses illustrate the mechanical, microenvironmental, and microglial modulation of activated astrocytes in glaucoma. Findings relevant to the possible mechanism of the astrocyte death in advanced glaucoma are also emerging. Ultimately, a better understanding of glaucomatous glial response may lead to glia-targeting neuroprotection in the future.
中文翻译:

基于神经胶质的视神经乳头中青光眼神经视网膜边缘变薄的解释。
神经视网膜边缘变薄(NRR)是青光眼性视盘改变的特征。但是,轮辋变薄的确切机理尚未完全阐明。这项审查侧重于胶质建筑在青光眼性NRR变薄形成中的结构作用。NRR是胶质框架结构,具有蜂窝状几何结构和沿横向平面机械增强的星形胶质细胞过程。当神经损伤选择性地牵涉神经元并保留神经胶质时,组织的总体结构得以保留。胶质细胞结构的紊乱和丧失是青光眼中视神经头(ONH)重塑的两个标志,导致轴突丧失时NRR组织变薄。这与大多数具有视盘苍白的非青光眼性视神经病变相反,在视神经苍白的情况下,神经胶质结构的肥大与看似缺乏的视盘拔罐有关。动脉前部缺血性视神经病变是ONH组织的全坏死导致NRR变薄的例外。轻度缺血表明非神经性前部缺血性视神经病变中胶质细胞的选择性神经元丢失。生物学原因是由损伤的部位,类型和严重程度决定的异质性神经胶质反应。神经胶质的解释解释了细胞变化如何构成临床发现的基础。关于神经胶质反应的最新理解说明了青光眼中活化星形胶质细胞的机械,微环境和微胶质调节。与晚期青光眼星形胶质细胞死亡的可能机制有关的发现也正在出现。最终,对青光眼神经胶质反应的更好理解可能会导致将来针对胶质细胞的神经保护作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号