当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Are free radicals actually responsible for enhanced oxidation of contaminants by Cr(VI) in the presence of bisulfite?
Chemosphere ( IF 8.8 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.chemosphere.2020.126000 Yuan Gao 1 , Han-Ping Pan 1 , Yang Zhou 1 , Zhen Wang 2 , Su-Yan Pang 3 , Chao-Ting Guan 2 , Yong-Ming Shen 4 , Jin Jiang 1
Chemosphere ( IF 8.8 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.chemosphere.2020.126000 Yuan Gao 1 , Han-Ping Pan 1 , Yang Zhou 1 , Zhen Wang 2 , Su-Yan Pang 3 , Chao-Ting Guan 2 , Yong-Ming Shen 4 , Jin Jiang 1
Affiliation
|
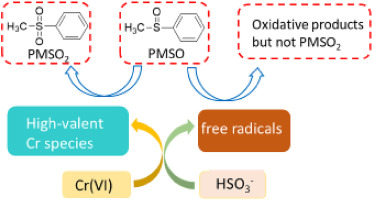
Recently, the technology for the remediation of Cr(VI) pollutant via bisulfite has been found to be effective for fast elimination of co-contaminants especially in acidic solution, where free radicals (i.e., sulfate and/or hydroxyl radicals) are proposed to act as dominant oxidants. Here, it was demonstrated that high-valent Cr intermediate played a primary role in the Cr(VI)/bisulfite system through applying methyl phenyl sulfoxide (PMSO) as a probe. PMSO was effectively transformed in the Cr(VI)/bisulfite system with appreciable generation of methyl phenyl sulfone (PMSO2) product, while PMSO was oxidized by free radicals to hydroxylated and/or polymeric products rather than PMSO2. The involvement of high-valent Cr species was further supported by the formation of 18O-labeled PMSO2 in 18O labeling experiments, where the incorporation of 18O from solvent water H218O into PMSO2 was likely resulted from competitive oxygen exchange of Cr-oxo species with water. The relative contribution of high valent Cr species versus free radicals was evaluated based on the yield of PMSO2, which was dependent on the solution chemistry such as [Cr(VI)]:[bisulfite] ratio and dissolved oxygen. This work advances the understanding of chromium chemistry involved in the Cr(VI)/bisulfite system. These findings have important implications on the application of this "waste control by waste" technology for environmental decontamination.
中文翻译:

在亚硫酸氢盐存在下,自由基是否真正导致六价铬(Cr)增强污染物的氧化作用?
最近,已发现通过亚硫酸氢盐修复Cr(VI)污染物的技术可有效快速消除共污染物,尤其是在酸性溶液中,其中建议自由基(即硫酸盐和/或羟基自由基)起作用作为主要氧化剂。在此,通过使用甲基苯基亚砜(PMSO)作为探针,证明了高价铬中间体在Cr(VI)/亚硫酸氢盐体系中起主要作用。PMSO有效地转化为Cr(VI)/亚硫酸氢盐体系,并生成了甲基苯基砜(PMSO2)产品,而PMSO被自由基氧化为羟基化和/或聚合产物,而不是PMSO2。18O标记实验中18O标记的PMSO2的形成进一步支持了高价铬物质的参与,溶剂水H218O中的18O掺入PMSO2可能是由于Cr-oxo物种与水之间的竞争性氧交换所致。基于PMSO2的收率评估了高价Cr物种相对于自由基的相对贡献,而PMSO2的收率则取决于溶液化学性质,例如[Cr(VI)]:[亚硫酸氢盐]比和溶解氧。这项工作提高了对Cr(VI)/亚硫酸氢盐体系中涉及的铬化学的理解。这些发现对这种“废物控制废物”技术在环境净化中的应用具有重要意义。这取决于溶液化学性质,例如[Cr(VI)]:[亚硫酸氢盐]的比例和溶解氧。这项工作提高了对Cr(VI)/亚硫酸氢盐体系中涉及的铬化学的理解。这些发现对这种“废物控制废物”技术在环境净化中的应用具有重要意义。这取决于溶液化学性质,例如[Cr(VI)]:[亚硫酸氢盐]的比例和溶解氧。这项工作提高了对Cr(VI)/亚硫酸氢盐体系中涉及的铬化学的理解。这些发现对这种“废物控制废物”技术在环境净化中的应用具有重要意义。
更新日期:2020-01-23
中文翻译:

在亚硫酸氢盐存在下,自由基是否真正导致六价铬(Cr)增强污染物的氧化作用?
最近,已发现通过亚硫酸氢盐修复Cr(VI)污染物的技术可有效快速消除共污染物,尤其是在酸性溶液中,其中建议自由基(即硫酸盐和/或羟基自由基)起作用作为主要氧化剂。在此,通过使用甲基苯基亚砜(PMSO)作为探针,证明了高价铬中间体在Cr(VI)/亚硫酸氢盐体系中起主要作用。PMSO有效地转化为Cr(VI)/亚硫酸氢盐体系,并生成了甲基苯基砜(PMSO2)产品,而PMSO被自由基氧化为羟基化和/或聚合产物,而不是PMSO2。18O标记实验中18O标记的PMSO2的形成进一步支持了高价铬物质的参与,溶剂水H218O中的18O掺入PMSO2可能是由于Cr-oxo物种与水之间的竞争性氧交换所致。基于PMSO2的收率评估了高价Cr物种相对于自由基的相对贡献,而PMSO2的收率则取决于溶液化学性质,例如[Cr(VI)]:[亚硫酸氢盐]比和溶解氧。这项工作提高了对Cr(VI)/亚硫酸氢盐体系中涉及的铬化学的理解。这些发现对这种“废物控制废物”技术在环境净化中的应用具有重要意义。这取决于溶液化学性质,例如[Cr(VI)]:[亚硫酸氢盐]的比例和溶解氧。这项工作提高了对Cr(VI)/亚硫酸氢盐体系中涉及的铬化学的理解。这些发现对这种“废物控制废物”技术在环境净化中的应用具有重要意义。这取决于溶液化学性质,例如[Cr(VI)]:[亚硫酸氢盐]的比例和溶解氧。这项工作提高了对Cr(VI)/亚硫酸氢盐体系中涉及的铬化学的理解。这些发现对这种“废物控制废物”技术在环境净化中的应用具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号