当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydroacridines: Part 33. An experimental and DFT study of the 13 C NMR chemical shifts of the nitrosamines derived from the six stereoisomers of tetradecahydroacridine
Magnetic Resonance in Chemistry ( IF 2 ) Pub Date : 2020-01-22 , DOI: 10.1002/mrc.4946 Francisc Potmischil 1 , Mihaela Hillebrand 2 , Hermann Kalchhauser 3
Magnetic Resonance in Chemistry ( IF 2 ) Pub Date : 2020-01-22 , DOI: 10.1002/mrc.4946 Francisc Potmischil 1 , Mihaela Hillebrand 2 , Hermann Kalchhauser 3
Affiliation
|
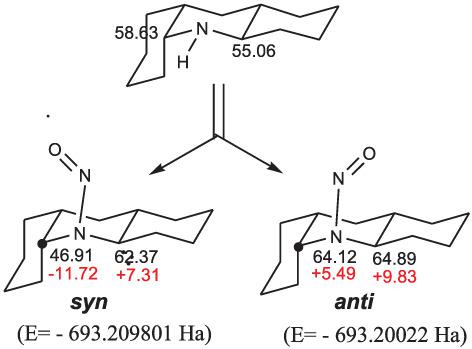
The paper presents the experimental and DFT‐calculated values of the 13C NMR chemical shifts of the six stereoisomers of tetradecahydroacridine and of the corresponding nitrosamines. Performing the DFT calculations using several combinations of functional and basis sets, it was found that the best experimental‐calculated agreement was obtained for OPBE/6–311++G (dp) method. Considering the effect of N‐nitrosation upon the 13C NMR chemical shifts of the C‐α carbons of secondary amines, it was found that if following nitrosation both C‐α carbons are shifted upfield or both are shifted downfield, then the resulted nitrosamine will have a sterically strained ─N═O group. If, however, one of the C‐α is shifted upfield and the other is shifted downfield, then the ─N═O group will be strain‐free or weakly strained. Our calculations predict strain energies of about 10–15 kcal mol−1 in the first case and ≈0–6 kcal mol−1 in the latter.
中文翻译:

氢吖啶:第 33 部分。十四氢吖啶的六种立体异构体衍生的亚硝胺的 13 C NMR 化学位移的实验和 DFT 研究
本文介绍了十四氢吖啶和相应亚硝胺的六种立体异构体的 13C NMR 化学位移的实验值和 DFT 计算值。使用函数和基组的几种组合执行 DFT 计算,发现 OPBE/6–311++G (dp) 方法获得了最佳的实验计算一致性。考虑到 N-亚硝化对仲胺的 C-α 碳的 13C NMR 化学位移的影响,发现如果在亚硝化之后两个 C-α 碳都向上场移动或都向下场移动,那么所得亚硝胺将具有空间应变的─N=O 基团。然而,如果 C-α 中的一个在场上移动而另一个在场下移动,那么 ─N=O 基团将是无应变或弱应变的。
更新日期:2020-01-22
中文翻译:

氢吖啶:第 33 部分。十四氢吖啶的六种立体异构体衍生的亚硝胺的 13 C NMR 化学位移的实验和 DFT 研究
本文介绍了十四氢吖啶和相应亚硝胺的六种立体异构体的 13C NMR 化学位移的实验值和 DFT 计算值。使用函数和基组的几种组合执行 DFT 计算,发现 OPBE/6–311++G (dp) 方法获得了最佳的实验计算一致性。考虑到 N-亚硝化对仲胺的 C-α 碳的 13C NMR 化学位移的影响,发现如果在亚硝化之后两个 C-α 碳都向上场移动或都向下场移动,那么所得亚硝胺将具有空间应变的─N=O 基团。然而,如果 C-α 中的一个在场上移动而另一个在场下移动,那么 ─N=O 基团将是无应变或弱应变的。

























 京公网安备 11010802027423号
京公网安备 11010802027423号