Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New regulatory mechanism-based inhibitors of aspartate transcarbamoylase for potential anticancer drug development.
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-22 , DOI: 10.1111/febs.15220 Zhen Lei 1 , Biying Wang 1 , Zhifang Lu 1 , Nan Wang 1, 2 , Hongwei Tan 1 , Jimin Zheng 1 , Zongchao Jia 3
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-22 , DOI: 10.1111/febs.15220 Zhen Lei 1 , Biying Wang 1 , Zhifang Lu 1 , Nan Wang 1, 2 , Hongwei Tan 1 , Jimin Zheng 1 , Zongchao Jia 3
Affiliation
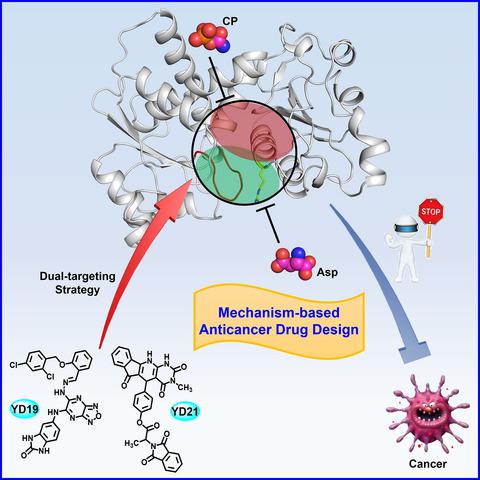
|
Aspartate transcarbamoylase (ATCase) is a key enzyme which regulates and catalyzes the second step of de novo pyrimidine synthesis in all organisms. Escherichia coli ATCase is a prototypic enzyme regulated by both product feedback and substrate cooperativity, whereas human ATCase is a potential anticancer target. Through structural and biochemical analyses, we revealed that R167/130's loop region in ATCase serves as a gatekeeper for the active site, playing a new and unappreciated regulatory role in the catalytic cycle of ATCase. Based on virtual compound screening simultaneously targeting the new regulatory region and active site of human ATCase, two compounds were identified to exhibit strong inhibition of ATCase activity, proliferation of multiple cancer cell lines, and growth of xenograft tumors. Our work has not only revealed a previously unknown regulatory region of ATCase that helps uncover the catalytic and regulatory mechanism of ATCase, but also successfully guided the identification of new ATCase inhibitors for anticancer drug development using a dual‐targeting strategy.
中文翻译:

新的基于调节机制的天冬氨酸转氨甲酰酶抑制剂,可用于潜在的抗癌药物开发。
天冬氨酸转氨甲酰酶(ATCase)是一种关键酶,它调节和催化所有生物中从头合成嘧啶的第二步。大肠杆菌ATCase是一种受产品反馈和底物协同作用调节的原型酶,而人ATCase是潜在的抗癌靶标。通过结构和生化分析,我们揭示了ATCase中的R167 / 130环区域充当了活性位点的守门人,在ATCase的催化循环中发挥了新的和未被理解的调节作用。基于同时靶向人类ATCase的新调控区和活性位点的虚拟化合物筛选,鉴定出两种化合物显示出对ATCase活性的强烈抑制,多种癌细胞系的增殖以及异种移植瘤的生长。我们的工作不仅揭示了以前未知的ATCase调控区域,有助于揭示ATCase的催化和调控机制,
更新日期:2020-01-22
中文翻译:

新的基于调节机制的天冬氨酸转氨甲酰酶抑制剂,可用于潜在的抗癌药物开发。
天冬氨酸转氨甲酰酶(ATCase)是一种关键酶,它调节和催化所有生物中从头合成嘧啶的第二步。大肠杆菌ATCase是一种受产品反馈和底物协同作用调节的原型酶,而人ATCase是潜在的抗癌靶标。通过结构和生化分析,我们揭示了ATCase中的R167 / 130环区域充当了活性位点的守门人,在ATCase的催化循环中发挥了新的和未被理解的调节作用。基于同时靶向人类ATCase的新调控区和活性位点的虚拟化合物筛选,鉴定出两种化合物显示出对ATCase活性的强烈抑制,多种癌细胞系的增殖以及异种移植瘤的生长。我们的工作不仅揭示了以前未知的ATCase调控区域,有助于揭示ATCase的催化和调控机制,



























 京公网安备 11010802027423号
京公网安备 11010802027423号