当前位置:
X-MOL 学术
›
Environ. Pollut.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sp1 participates in the cadmium-induced imbalance of the placental glucocorticoid barrier by suppressing 11β-HSD2 expression.
Environmental Pollution ( IF 8.9 ) Pub Date : 2020-01-22 , DOI: 10.1016/j.envpol.2020.113976 Fengyun Fan 1 , Wanting Shen 2 , Sisi Wu 1 , Na Chen 1 , Xia Tong 1 , Fan Wang 1 , Qiong Zhang 3
Environmental Pollution ( IF 8.9 ) Pub Date : 2020-01-22 , DOI: 10.1016/j.envpol.2020.113976 Fengyun Fan 1 , Wanting Shen 2 , Sisi Wu 1 , Na Chen 1 , Xia Tong 1 , Fan Wang 1 , Qiong Zhang 3
Affiliation
|
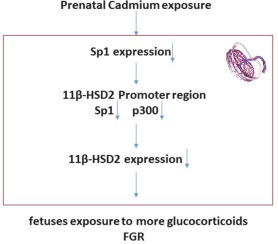
Cadmium (Cd) is widely present in the environment as a heavy metal poison. Prenatal Cd exposure can damage the placental glucocorticoid barrier, leading to foetal growth restriction (FGR), but the molecular mechanism is unknown. We aimed to study the effects of prenatal Cd exposure on 11β-HSD2 and its possible involvement in Cd induced damage in the placental glucocorticoid barrier. Pregnant rats were treated with CdCl2 (1.0 mg/kg/day) by gavage from gestational day (GD) 9-19. Maternal exposure to Cd increased the FGR rate of the offspring, and the levels of corticosterone in the placenta, maternal and foetal serum. Further in vitro experiments with placenta or JEG3 cells indicated that Cd was able to decrease 11β-HSD2 and Sp1 expression in trophoblast cells but did not affect 11β-HSD1. Additionally, decreased p300 and Sp1 enrichment at the 11β-HSD2 promoter region was observed in the cells treated with Cd. Decreasing or increasing Sp1 expression accordingly inhibited or promoted the expression of 11β-HSD2 and further decreased or increased p300 and Sp1 enrichment at the 11β-HSD2 promoter region. In conclusion, Cd inhibits the expression of 11β-HSD2 by affecting the binding of p300 to 11β-HSD2 via a decrease in Sp1 expression, which damages the placental glucocorticoid barrier and exposes the foetus to excessive glucocorticoids, resulting in FGR. These findings reveal a possible underlying molecular mechanism by which Cd exposure leads to FGR.
中文翻译:

Sp1通过抑制11β-HSD2表达而参与镉诱导的胎盘糖皮质激素屏障失衡。
镉(Cd)作为重金属毒物广泛存在于环境中。产前Cd暴露可破坏胎盘糖皮质激素屏障,导致胎儿生长受限(FGR),但分子机制尚不清楚。我们旨在研究产前Cd暴露对11β-HSD2的影响及其可能参与Cd诱导的胎盘糖皮质激素屏障损害。从妊娠日(GD)9-19开始,通过管饲法用CdCl2(1.0 mg / kg /天)处理妊娠大鼠。孕妇接触镉会增加后代的FGR率,并增加胎盘,母体和胎儿血清中的皮质酮水平。进一步的胎盘或JEG3细胞体外实验表明,Cd能够降低滋养细胞中11β-HSD2和Sp1的表达,但不影响11β-HSD1。另外,在用Cd处理的细胞中观察到p300和Sp1在11β-HSD2启动子区域的富集水平降低。Sp1表达的减少或增加相应地抑制或促进了11β-HSD2的表达,并进一步减少或增加了11β-HSD2启动子区域的p300和Sp1富集。总之,Cd通过Sp1的表达下降来影响p300与11β-HSD2的结合,从而抑制11β-HSD2的表达,从而损害胎盘糖皮质激素屏障并使胎儿暴露于过量的糖皮质激素,从而导致FGR。这些发现揭示了镉暴露可能导致FGR的潜在分子机制。Sp1表达的减少或增加相应地抑制或促进了11β-HSD2的表达,并进一步减少或增加了11β-HSD2启动子区域的p300和Sp1富集。总之,Cd通过Sp1的表达下降来影响p300与11β-HSD2的结合,从而抑制11β-HSD2的表达,从而损害胎盘糖皮质激素屏障并使胎儿暴露于过量的糖皮质激素,从而导致FGR。这些发现揭示了镉暴露导致FGR的潜在分子机制。Sp1表达的减少或增加相应地抑制或促进了11β-HSD2的表达,并进一步减少或增加了11β-HSD2启动子区域的p300和Sp1富集。总之,Cd通过Sp1表达的降低影响p300与11β-HSD2的结合,从而抑制11β-HSD2的表达,从而损害胎盘糖皮质激素屏障并使胎儿暴露于过量的糖皮质激素,从而导致FGR。这些发现揭示了镉暴露导致FGR的潜在分子机制。
更新日期:2020-01-22
中文翻译:

Sp1通过抑制11β-HSD2表达而参与镉诱导的胎盘糖皮质激素屏障失衡。
镉(Cd)作为重金属毒物广泛存在于环境中。产前Cd暴露可破坏胎盘糖皮质激素屏障,导致胎儿生长受限(FGR),但分子机制尚不清楚。我们旨在研究产前Cd暴露对11β-HSD2的影响及其可能参与Cd诱导的胎盘糖皮质激素屏障损害。从妊娠日(GD)9-19开始,通过管饲法用CdCl2(1.0 mg / kg /天)处理妊娠大鼠。孕妇接触镉会增加后代的FGR率,并增加胎盘,母体和胎儿血清中的皮质酮水平。进一步的胎盘或JEG3细胞体外实验表明,Cd能够降低滋养细胞中11β-HSD2和Sp1的表达,但不影响11β-HSD1。另外,在用Cd处理的细胞中观察到p300和Sp1在11β-HSD2启动子区域的富集水平降低。Sp1表达的减少或增加相应地抑制或促进了11β-HSD2的表达,并进一步减少或增加了11β-HSD2启动子区域的p300和Sp1富集。总之,Cd通过Sp1的表达下降来影响p300与11β-HSD2的结合,从而抑制11β-HSD2的表达,从而损害胎盘糖皮质激素屏障并使胎儿暴露于过量的糖皮质激素,从而导致FGR。这些发现揭示了镉暴露可能导致FGR的潜在分子机制。Sp1表达的减少或增加相应地抑制或促进了11β-HSD2的表达,并进一步减少或增加了11β-HSD2启动子区域的p300和Sp1富集。总之,Cd通过Sp1的表达下降来影响p300与11β-HSD2的结合,从而抑制11β-HSD2的表达,从而损害胎盘糖皮质激素屏障并使胎儿暴露于过量的糖皮质激素,从而导致FGR。这些发现揭示了镉暴露导致FGR的潜在分子机制。Sp1表达的减少或增加相应地抑制或促进了11β-HSD2的表达,并进一步减少或增加了11β-HSD2启动子区域的p300和Sp1富集。总之,Cd通过Sp1表达的降低影响p300与11β-HSD2的结合,从而抑制11β-HSD2的表达,从而损害胎盘糖皮质激素屏障并使胎儿暴露于过量的糖皮质激素,从而导致FGR。这些发现揭示了镉暴露导致FGR的潜在分子机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号