Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
BRCA1 protects cardiac microvascular endothelial cells against irradiation by regulating p21-mediated cell cycle arrest.
Life Sciences ( IF 6.1 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.lfs.2020.117342 Zhi-Min Zeng 1 , Hai-Yang Du 1 , Le Xiong 1 , Xiao-Li Zeng 2 , Peng Zhang 3 , Jing Cai 2 , Long Huang 1 , An-Wen Liu 1
Life Sciences ( IF 6.1 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.lfs.2020.117342 Zhi-Min Zeng 1 , Hai-Yang Du 1 , Le Xiong 1 , Xiao-Li Zeng 2 , Peng Zhang 3 , Jing Cai 2 , Long Huang 1 , An-Wen Liu 1
Affiliation
|
AIMS
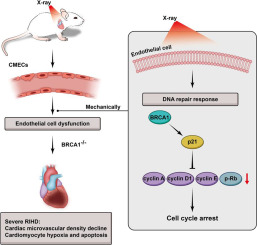
Microvascular endothelial cell dysfunction is a leading cause of radiation-induced heart disease (RIHD). BRCA1 plays an important role in DNA damage repair. The study aims to explore the effect of BRCA1 in endothelial cells involved in RIHD.
MATERIALS AND METHODS
BRCA1 and p21 expression were detected in human umbilical vein endothelial cells (HUVECs) and in mouse heart tissue after irradiation exposure. The effects of BRCA1 on cell proliferation, cell cycle and radiosensitivity were determined in HUVECs with overexpression and knockdown of BRCA1. A mouse model of RIHD was established. Heart damage was detected in C57BL/6J mice and endothelial cell specific knockout BRCA1 mice (EC-BRCA1-/-).
KEY FINDINGS
BRCA1 and p21 expression was significantly increased both in vitro and vivo response to irradiation. BRCA1 overexpression in endothelial cells enhanced cell growth and G1/S phase arrest, and the opposite results were observed in BRCA1 knockdown endothelial cells. BRCA1 downregulated endothelial cell cycle-related genes cyclin A, cyclin D1, cyclin E and p-Rb through increasing p21 expression, and HUVECs with BRCA1 gene knockdown were more sensitive to radiation. In vivo, a decrease in cardiac microvascular density, as well as cardiomyocyte hypoxia and apoptosis were observed in a time-dependent manner. EC-BRCA1-/- mice were more prone to severe RIHD than EC-BRCA1+/- mice after 16Gy radiation exposure due to endothelial dysfunction caused by loss of BRCA1, and p21 was declined in EC-BRCA1-/- mice heart.
SIGNIFICANCE
These findings indicate that BRCA1 plays a protective role in RIHD by regulating endothelial cell cycle arrest mediated by p21 signal.
中文翻译:

BRCA1通过调节p21介导的细胞周期停滞来保护心脏微血管内皮细胞免受辐射。
AIMS微血管内皮细胞功能障碍是放射诱发性心脏病(RIHD)的主要原因。BRCA1在DNA损伤修复中起重要作用。该研究旨在探讨BRCA1在RIHD参与的内皮细胞中的作用。材料与方法在辐射照射后的人脐静脉内皮细胞(HUVEC)和小鼠心脏组织中检测到BRCA1和p21表达。在具有BRCA1过度表达和敲低的HUVEC中确定了BRCA1对细胞增殖,细胞周期和放射敏感性的影响。建立了RIHD的小鼠模型。在C57BL / 6J小鼠和内皮细胞特异性敲除BRCA1小鼠(EC-BRCA1-/-)中检测到心脏损伤。主要发现BRCA1和p21表达在体外和体内对辐射的反应中均显着增加。BRCA1在内皮细胞中的过表达增强了细胞的生长和G1 / S期阻滞,在BRCA1抑制的内皮细胞中观察到相反的结果。BRCA1通过增加p21表达来下调内皮细胞周期相关基因cyclin A,cyclin D1,cyclin E和p-Rb,而具有BRCA1基因敲低功能的HUVEC对辐射更敏感。在体内,以时间依赖性方式观察到心脏微血管密度的降低以及心肌细胞的缺氧和凋亡。由于BRCA1丢失引起的内皮功能障碍,在16Gy辐射照射后,EC-BRCA1-/-小鼠比EC-BRCA1 +/-小鼠更容易出现严重的RIHD,并且在EC-BRCA1-/-小鼠心脏中p21下降。
更新日期:2020-01-22
中文翻译:

BRCA1通过调节p21介导的细胞周期停滞来保护心脏微血管内皮细胞免受辐射。
AIMS微血管内皮细胞功能障碍是放射诱发性心脏病(RIHD)的主要原因。BRCA1在DNA损伤修复中起重要作用。该研究旨在探讨BRCA1在RIHD参与的内皮细胞中的作用。材料与方法在辐射照射后的人脐静脉内皮细胞(HUVEC)和小鼠心脏组织中检测到BRCA1和p21表达。在具有BRCA1过度表达和敲低的HUVEC中确定了BRCA1对细胞增殖,细胞周期和放射敏感性的影响。建立了RIHD的小鼠模型。在C57BL / 6J小鼠和内皮细胞特异性敲除BRCA1小鼠(EC-BRCA1-/-)中检测到心脏损伤。主要发现BRCA1和p21表达在体外和体内对辐射的反应中均显着增加。BRCA1在内皮细胞中的过表达增强了细胞的生长和G1 / S期阻滞,在BRCA1抑制的内皮细胞中观察到相反的结果。BRCA1通过增加p21表达来下调内皮细胞周期相关基因cyclin A,cyclin D1,cyclin E和p-Rb,而具有BRCA1基因敲低功能的HUVEC对辐射更敏感。在体内,以时间依赖性方式观察到心脏微血管密度的降低以及心肌细胞的缺氧和凋亡。由于BRCA1丢失引起的内皮功能障碍,在16Gy辐射照射后,EC-BRCA1-/-小鼠比EC-BRCA1 +/-小鼠更容易出现严重的RIHD,并且在EC-BRCA1-/-小鼠心脏中p21下降。



























 京公网安备 11010802027423号
京公网安备 11010802027423号