当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of fulvic acid and montmorillonite colloids at different concentrations on Cd(II) sorption onto nano-hydroxyapatite.
Chemosphere ( IF 8.8 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.chemosphere.2020.125992 Mengmeng Wu 1 , Limei Mo 1 , Erping Bi 1
Chemosphere ( IF 8.8 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.chemosphere.2020.125992 Mengmeng Wu 1 , Limei Mo 1 , Erping Bi 1
Affiliation
|
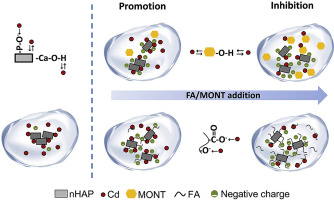
Natural colloids can influence the binding mechanisms between nano-hydroxyapatite (nHAP) and Cd(II). In this study, the effects of organic and inorganic natural colloids on Cd(II) sorption onto nHAP were compared. Different experimental approaches combined with the additivity model and the Extended-Derjaguin-Landau-Verwey-Overbeek model were used to quantify the distribution of Cd(II) in the systems of nHAP and natural colloid, and the interaction energy between particles. The results showed that both fulvic acid (FA) and montmorillonite colloid (MONT) had the promotion and inhibition effects on Cd(II) sorption onto nHAP. Coexistence of FA or MONT could stabilize nHAP particles. FA could adsorb onto nHAP particle surface via carboxylic and phenolic groups, which increased nHAP electronegativity and formed steric resistance effect. Coexistence of MONT mainly increased nHAP electronegativity. These effects prevented the reduction of the specific surface area of nHAP particles and increased the Cd(II) sorption onto nHAP. However, the inhibition effect on Cd(II) sorption was enhanced with increasing concentration of FA or MONT because more soluble FA-Cd or suspended MONT-Cd complexes formed in the system. In nHAP-FA-Cd systems, the Cd(II) sorption onto FA was well predicted but that onto solid phase was underestimated by the additivity model. In nHAP-MONT-Cd systems, Cd(II) sorbed onto mixtures of nHAP and MONT was well described by the additive model. The findings of this study can help to understand the fate of Cd(II) in natural water and soil.
中文翻译:

富里酸和蒙脱石胶体对纳米羟基磷灰石吸附Cd(II)的影响。
天然胶体可以影响纳米羟基磷灰石(nHAP)和Cd(II)之间的结合机制。在这项研究中,比较了有机和无机天然胶体对Cd(II)在nHAP上的吸附作用。结合加性模型和Extended-Derjaguin-Landau-Verwey-Overbeek模型,采用不同的实验方法对Cd(II)在nHAP和天然胶体体系中的分布以及颗粒之间的相互作用能进行了定量。结果表明,富里酸(FA)和蒙脱石胶体(MONT)均具有促进和抑制Cd(II)吸附在nHAP上的作用。FA或MONT的共存可稳定nHAP颗粒。FA可以通过羧基和酚基团吸附到nHAP颗粒表面,从而增加nHAP的电负性并形成位阻效应。MONT的共存主要增加nHAP电负性。这些效果阻止了nHAP颗粒比表面积的减少,并增加了Cd(II)在nHAP上的吸附。但是,随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用会增强,因为系统中会形成更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。这些效果阻止了nHAP颗粒比表面积的减少,并增加了Cd(II)在nHAP上的吸附。但是,随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用会增强,因为系统中会形成更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。这些效果阻止了nHAP颗粒比表面积的减少,并增加了Cd(II)在nHAP上的吸附。但是,随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用会增强,因为系统中会形成更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用增强,因为系统中形成了更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用增强,因为系统中形成了更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。
更新日期:2020-01-22
中文翻译:

富里酸和蒙脱石胶体对纳米羟基磷灰石吸附Cd(II)的影响。
天然胶体可以影响纳米羟基磷灰石(nHAP)和Cd(II)之间的结合机制。在这项研究中,比较了有机和无机天然胶体对Cd(II)在nHAP上的吸附作用。结合加性模型和Extended-Derjaguin-Landau-Verwey-Overbeek模型,采用不同的实验方法对Cd(II)在nHAP和天然胶体体系中的分布以及颗粒之间的相互作用能进行了定量。结果表明,富里酸(FA)和蒙脱石胶体(MONT)均具有促进和抑制Cd(II)吸附在nHAP上的作用。FA或MONT的共存可稳定nHAP颗粒。FA可以通过羧基和酚基团吸附到nHAP颗粒表面,从而增加nHAP的电负性并形成位阻效应。MONT的共存主要增加nHAP电负性。这些效果阻止了nHAP颗粒比表面积的减少,并增加了Cd(II)在nHAP上的吸附。但是,随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用会增强,因为系统中会形成更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。这些效果阻止了nHAP颗粒比表面积的减少,并增加了Cd(II)在nHAP上的吸附。但是,随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用会增强,因为系统中会形成更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。这些效果阻止了nHAP颗粒比表面积的减少,并增加了Cd(II)在nHAP上的吸附。但是,随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用会增强,因为系统中会形成更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用增强,因为系统中形成了更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。随着FA或MONT浓度的增加,对Cd(II)吸附的抑制作用增强,因为系统中形成了更多的可溶性FA-Cd或悬浮的MONT-Cd复合物。在nHAP-FA-Cd系统中,可以很好地预测Cd(II)在FA上的吸附,但通过加和模型低估了Cd(II)在固相上的吸附。在nHAP-MONT-Cd系统中,添加剂模型很好地描述了吸附在nHAP和MONT混合物上的Cd(II)。这项研究的结果可以帮助理解天然水和土壤中Cd(II)的命运。



























 京公网安备 11010802027423号
京公网安备 11010802027423号