当前位置:
X-MOL 学术
›
PLOS Genet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Roles of Candida albicans Mig1 and Mig2 in glucose repression, pathogenicity traits, and SNF1 essentiality.
PLOS Genetics ( IF 4.5 ) Pub Date : 2020-01-21 , DOI: 10.1371/journal.pgen.1008582 Katherine Lagree 1 , Carol A Woolford 1 , Manning Y Huang 1 , Gemma May 1 , C Joel McManus 1 , Norma V Solis 2, 3 , Scott G Filler 2, 3 , Aaron P Mitchell 1, 4
PLOS Genetics ( IF 4.5 ) Pub Date : 2020-01-21 , DOI: 10.1371/journal.pgen.1008582 Katherine Lagree 1 , Carol A Woolford 1 , Manning Y Huang 1 , Gemma May 1 , C Joel McManus 1 , Norma V Solis 2, 3 , Scott G Filler 2, 3 , Aaron P Mitchell 1, 4
Affiliation
|
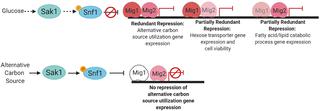
Metabolic adaptation is linked to the ability of the opportunistic pathogen Candida albicans to colonize and cause infection in diverse host tissues. One way that C. albicans controls its metabolism is through the glucose repression pathway, where expression of alternative carbon source utilization genes is repressed in the presence of its preferred carbon source, glucose. Here we carry out genetic and gene expression studies that identify transcription factors Mig1 and Mig2 as mediators of glucose repression in C. albicans. The well-studied Mig1/2 orthologs ScMig1/2 mediate glucose repression in the yeast Saccharomyces cerevisiae; our data argue that C. albicans Mig1/2 function similarly as repressors of alternative carbon source utilization genes. However, Mig1/2 functions have several distinctive features in C. albicans. First, Mig1 and Mig2 have more co-equal roles in gene regulation than their S. cerevisiae orthologs. Second, Mig1 is regulated at the level of protein accumulation, more akin to ScMig2 than ScMig1. Third, Mig1 and Mig2 are together required for a unique aspect of C. albicans biology, the expression of several pathogenicity traits. Such Mig1/2-dependent traits include the abilities to form hyphae and biofilm, tolerance of cell wall inhibitors, and ability to damage macrophage-like cells and human endothelial cells. Finally, Mig1 is required for a puzzling feature of C. albicans biology that is not shared with S. cerevisiae: the essentiality of the Snf1 protein kinase, a central eukaryotic carbon metabolism regulator. Our results integrate Mig1 and Mig2 into the C. albicans glucose repression pathway and illuminate connections among carbon control, pathogenicity, and Snf1 essentiality.
中文翻译:

白色念珠菌Mig1和Mig2在葡萄糖抑制,致病性状和SNF1必需性中的作用。
代谢适应与机会病原体白色念珠菌在各种宿主组织中定植并引起感染的能力有关。白色念珠菌控制其代谢的一种方式是通过葡萄糖抑制途径,其中在存在其优选碳源葡萄糖的情况下抑制了替代碳源利用基因的表达。在这里,我们进行了遗传和基因表达研究,确定了转录因子Mig1和Mig2作为白色念珠菌中葡萄糖抑制的介质。深入研究的Mig1 / 2直系同源物ScMig1 / 2介导了酿酒酵母中的葡萄糖阻抑作用;我们的数据认为白色念珠菌Mig1 / 2的功能与替代碳源利用基因的阻遏物相似。但是,Mig1 / 2功能在白色念珠菌中具有几个独特的特征。第一,Mig1和Mig2在基因调控中比酿酒酵母直系同源基因具有更多的同等作用。其次,Mig1在蛋白质积累水平受到调控,与ScMig1相似,更类似于ScMig2。第三,Mig1和Mig2是白色念珠菌生物学独特方面(几个致病性状的表达)所必需的。这种依赖Mig1 / 2的性状包括形成菌丝和生物膜的能力,对细胞壁抑制剂的耐受性以及对巨噬细胞样细胞和人内皮细胞的损害能力。最后,Mig1是白色念珠菌生物学的一个令人困惑的特征所必需的,而这个特征与酿酒酵母不共有:Snf1蛋白激酶(一种核心的真核碳代谢调节剂)的必要性。我们的结果将Mig1和Mig2整合到白色念珠菌的葡萄糖阻遏途径中,并阐明了碳控制之间的联系,
更新日期:2020-02-18
中文翻译:

白色念珠菌Mig1和Mig2在葡萄糖抑制,致病性状和SNF1必需性中的作用。
代谢适应与机会病原体白色念珠菌在各种宿主组织中定植并引起感染的能力有关。白色念珠菌控制其代谢的一种方式是通过葡萄糖抑制途径,其中在存在其优选碳源葡萄糖的情况下抑制了替代碳源利用基因的表达。在这里,我们进行了遗传和基因表达研究,确定了转录因子Mig1和Mig2作为白色念珠菌中葡萄糖抑制的介质。深入研究的Mig1 / 2直系同源物ScMig1 / 2介导了酿酒酵母中的葡萄糖阻抑作用;我们的数据认为白色念珠菌Mig1 / 2的功能与替代碳源利用基因的阻遏物相似。但是,Mig1 / 2功能在白色念珠菌中具有几个独特的特征。第一,Mig1和Mig2在基因调控中比酿酒酵母直系同源基因具有更多的同等作用。其次,Mig1在蛋白质积累水平受到调控,与ScMig1相似,更类似于ScMig2。第三,Mig1和Mig2是白色念珠菌生物学独特方面(几个致病性状的表达)所必需的。这种依赖Mig1 / 2的性状包括形成菌丝和生物膜的能力,对细胞壁抑制剂的耐受性以及对巨噬细胞样细胞和人内皮细胞的损害能力。最后,Mig1是白色念珠菌生物学的一个令人困惑的特征所必需的,而这个特征与酿酒酵母不共有:Snf1蛋白激酶(一种核心的真核碳代谢调节剂)的必要性。我们的结果将Mig1和Mig2整合到白色念珠菌的葡萄糖阻遏途径中,并阐明了碳控制之间的联系,



























 京公网安备 11010802027423号
京公网安备 11010802027423号