当前位置:
X-MOL 学术
›
Lancet Infect Dis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study.
The Lancet Infectious Diseases ( IF 56.3 ) Pub Date : 2020-01-21 , DOI: 10.1016/s1473-3099(19)30584-5 David Liebowitz 1 , Keith Gottlieb 1 , Nikita S Kolhatkar 1 , Shaily J Garg 1 , Jason M Asher 2 , Jonathan Nazareno 1 , Kenneth Kim 3 , David R McIlwain 4 , Sean N Tucker 1
The Lancet Infectious Diseases ( IF 56.3 ) Pub Date : 2020-01-21 , DOI: 10.1016/s1473-3099(19)30584-5 David Liebowitz 1 , Keith Gottlieb 1 , Nikita S Kolhatkar 1 , Shaily J Garg 1 , Jason M Asher 2 , Jonathan Nazareno 1 , Kenneth Kim 3 , David R McIlwain 4 , Sean N Tucker 1
Affiliation
|
BACKGROUND
Influenza is an important public health problem and existing vaccines are not completely protective. New vaccines that protect by alternative mechanisms are needed to improve efficacy of influenza vaccines. In 2015, we did a phase 1 trial of an oral influenza vaccine, VXA-A1.1. A favourable safety profile and robust immunogenicity results in that trial supported progression of the vaccine to the current phase 2 trial. The aim of this study was to evaluate efficacy of the vaccine in a human influenza challenge model.
METHODS
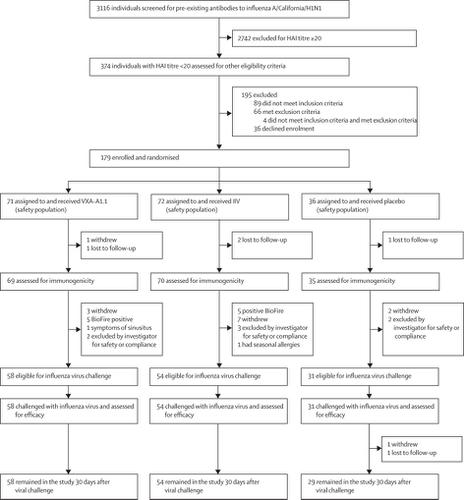
We did a single-site, placebo-controlled and active-controlled, phase 2 study at WCCT Global, Costa Mesa, CA, USA. Eligible individuals had an initial A/California/H1N1 haemagglutination inhibition titre of less than 20 and were aged 18-49 years and in good health. Individuals were randomly assigned (2:2:1) to receive a single immunisation of either 1011 infectious units of VXA-A1.1 (a monovalent tablet vaccine) orally, a full human dose of quadrivalent inactivated influenza vaccine (IIV) via intramuscular injection, or matched placebo. Randomisation was done by computer-generated assignments with block size of five. An unmasked pharmacist provided the appropriate vaccines and placebos to the administrating nurse. Individuals receiving the treatments, investigators, and staff were all masked to group assignments. 90 days after immunisation, individuals without clinically significant symptoms or signs of influenza, an oral temperature of higher than 37·9°C, a positive result for respiratory viral shedding on a Biofire test, and any investigator-assessed contraindications were challenged intranasally with 0·5 mL wild-type A/CA/like(H1N1)pdm09 influenza virus. The primary outcomes were safety, which was assessed in all immunised participants through 365 days, and influenza-positive illness after viral challenge, which was assessed in individuals that received the viral challenge and the required number of assessments post viral challenge. This trial is registered with ClinicalTrials.gov, number NCT02918006.
RESULTS
Between Aug 31, 2016, and Jan 23, 2017, 374 individuals were assessed for eligibility, of whom 179 were randomly assigned to receive either VXA-A1.1 (n=71 [one individual did not provide a diary card, thus the solicited events were assessed in 70 individuals]), IIV (n=72), or placebo (n=36). Between Dec 2, 2016, and April 26, 2017, 143 eligible individuals (58 in the VXA-A1.1 group, 54 in the IIV group, and 31 in the placebo group) were challenged with influenza virus. VXA-A1.1 was well tolerated with no serious or medically significant adverse events. The most prevalent solicited adverse events for each of the treatment groups after immunisation were headache in the VXA-A1.1 (in five [7%] of 70 participants) and placebo (in seven [19%] of 36 participants) groups and tenderness at injection site in the IIV group (in 19 [26%] of 72 participants) Influenza-positive illness after challenge was detected in 17 (29%) of 58 individuals in the VXA-A1.1 group, 19 (35%) of 54 in the IIV group, and 15 (48%) of 31 in the placebo group.
INTERPRETATION
Orally administered VXA-A1.1 was well tolerated and generated protective immunity against virus shedding, similar to a licensed intramuscular IIV. These results represent a major step forward in developing a safe and effective oral influenza vaccine.
FUNDING
Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, and Biomedical Advanced Research and Development Authority.
中文翻译:

口服流感疫苗的功效,免疫原性和安全性:安慰剂对照和活性对照的2期人类攻击研究。
背景技术流行性感冒是重要的公共卫生问题,并且现有的疫苗不能完全起到保护作用。需要通过替代机制保护的新疫苗来提高流感疫苗的效力。2015年,我们进行了口服流感疫苗VXA-A1.1的1期试验。良好的安全性和强大的免疫原性导致该试验支持疫苗发展至目前的2期试验。这项研究的目的是评估疫苗在人流感挑战模型中的功效。方法我们在美国加利福尼亚州科斯塔梅萨市的WCCT Global进行了单部位,安慰剂对照和活性对照的2期研究。符合条件的个体的初始A /加利福尼亚/ H1N1血凝抑制滴度小于20,年龄在18-49岁,身体健康。个体被随机分配(2:2:1)口服接受1011个VXA-A1.1感染单位(单价片剂疫苗)的单次免疫,通过肌肉注射或全剂量的人类四价灭活流感疫苗(IIV)或匹配的安慰剂。随机化是通过计算机生成的作业进行的,每个作业的块大小为5。一位不为人所知的药剂师向主管护士提供了适当的疫苗和安慰剂。接受治疗的人员,研究人员和工作人员都被掩盖在小组任务中。免疫90天后,没有临床上明显的流感症状或体征的人,口腔温度高于37·9°C,Biofire测试显示呼吸道病毒脱落为阳性结果,并以0·5 mL野生型A / CA / like(H1N1)pdm09流感病毒鼻内攻击所有研究者评估的禁忌症。主要结果是安全性(在365天之内对所有免疫接种的参与者进行了评估),以及病毒攻击后的流感阳性疾病(在接受病毒攻击的个体中进行了评估,并要求在病毒攻击后进行必要的评估次数)。该试验已在ClinicalTrials.gov上注册,编号为NCT02918006。结果在2016年8月31日至2017年1月23日之间,评估了374个人的资格,其中179人被随机分配接受任一VXA-A1.1(n = 71 [一个人没有提供日记卡,因此在70位个体中评估了被征集的事件],IIV(n = 72)或安慰剂(n = 36)。在2016年12月2日至2017年4月26日之间,143名合格个体(VXA-A1.1组为58,IIV组为54,安慰剂组为31)受到了流感病毒的攻击。VXA-A1.1具有良好的耐受性,没有严重或医学上显着的不良事件。免疫后每个治疗组引起的最普遍的不良事件是VXA-A1.1组(70名受试者中的5 [7%])和安慰剂组(36名受试者中的7 [19%])的头痛和压痛在IIV组的注射部位(72名参与者中的19名[26%])中,在VXA-A1.1组的58名个体中有17名(29%)检测出攻击后的流感阳性疾病,其中19名(35%)被检测出IIV组为54例,安慰剂组为31例中的15例(48%)。解释口服VXA-A1.1具有良好的耐受性,并具有针对病毒脱落的保护性免疫力,类似于获得许可的肌肉注射IIV。这些结果代表了开发安全有效的口服流感疫苗的重要一步。筹资卫生和公共服务部,准备和响应助理部长办公室和生物医学高级研究与开发管理局。
更新日期:2020-01-21
中文翻译:

口服流感疫苗的功效,免疫原性和安全性:安慰剂对照和活性对照的2期人类攻击研究。
背景技术流行性感冒是重要的公共卫生问题,并且现有的疫苗不能完全起到保护作用。需要通过替代机制保护的新疫苗来提高流感疫苗的效力。2015年,我们进行了口服流感疫苗VXA-A1.1的1期试验。良好的安全性和强大的免疫原性导致该试验支持疫苗发展至目前的2期试验。这项研究的目的是评估疫苗在人流感挑战模型中的功效。方法我们在美国加利福尼亚州科斯塔梅萨市的WCCT Global进行了单部位,安慰剂对照和活性对照的2期研究。符合条件的个体的初始A /加利福尼亚/ H1N1血凝抑制滴度小于20,年龄在18-49岁,身体健康。个体被随机分配(2:2:1)口服接受1011个VXA-A1.1感染单位(单价片剂疫苗)的单次免疫,通过肌肉注射或全剂量的人类四价灭活流感疫苗(IIV)或匹配的安慰剂。随机化是通过计算机生成的作业进行的,每个作业的块大小为5。一位不为人所知的药剂师向主管护士提供了适当的疫苗和安慰剂。接受治疗的人员,研究人员和工作人员都被掩盖在小组任务中。免疫90天后,没有临床上明显的流感症状或体征的人,口腔温度高于37·9°C,Biofire测试显示呼吸道病毒脱落为阳性结果,并以0·5 mL野生型A / CA / like(H1N1)pdm09流感病毒鼻内攻击所有研究者评估的禁忌症。主要结果是安全性(在365天之内对所有免疫接种的参与者进行了评估),以及病毒攻击后的流感阳性疾病(在接受病毒攻击的个体中进行了评估,并要求在病毒攻击后进行必要的评估次数)。该试验已在ClinicalTrials.gov上注册,编号为NCT02918006。结果在2016年8月31日至2017年1月23日之间,评估了374个人的资格,其中179人被随机分配接受任一VXA-A1.1(n = 71 [一个人没有提供日记卡,因此在70位个体中评估了被征集的事件],IIV(n = 72)或安慰剂(n = 36)。在2016年12月2日至2017年4月26日之间,143名合格个体(VXA-A1.1组为58,IIV组为54,安慰剂组为31)受到了流感病毒的攻击。VXA-A1.1具有良好的耐受性,没有严重或医学上显着的不良事件。免疫后每个治疗组引起的最普遍的不良事件是VXA-A1.1组(70名受试者中的5 [7%])和安慰剂组(36名受试者中的7 [19%])的头痛和压痛在IIV组的注射部位(72名参与者中的19名[26%])中,在VXA-A1.1组的58名个体中有17名(29%)检测出攻击后的流感阳性疾病,其中19名(35%)被检测出IIV组为54例,安慰剂组为31例中的15例(48%)。解释口服VXA-A1.1具有良好的耐受性,并具有针对病毒脱落的保护性免疫力,类似于获得许可的肌肉注射IIV。这些结果代表了开发安全有效的口服流感疫苗的重要一步。筹资卫生和公共服务部,准备和响应助理部长办公室和生物医学高级研究与开发管理局。

























 京公网安备 11010802027423号
京公网安备 11010802027423号