Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.3 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.jphotochem.2020.112402 Sonam Shakya , Ishaat M. Khan , Musheer Ahmad
|
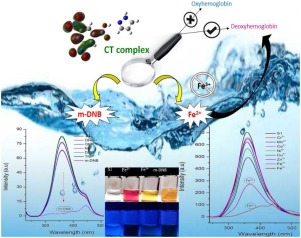
A cost-effective and easy to prepare colorimetric aqueous medium dual chemosensor (S1) as a charge transfer complex (CTC) in the form of precipitate was synthesized by one step procedure. S1 showed a highly selective fluorescence quenching response towards 1,3-dinitrobenzene over other nitro explosives with a low detection limit of 0.084 ppb and binding constant of 8.51 102 M−1. S1 also showed selective and discriminative detection between Fe2+ and Fe3+ state over other metal ions, which can be noticed in color difference among Fe2+ and Fe3+ by the naked eye. This discriminative detection of two states Fe ion was also found in human deoxyhemoglobin (HHb) and oxyhemoglobin (HbO2) with the binding constant of 132.53 M-1 and 26.46 M−1respectively. Molecular docking between S1 and hemoglobin provides free energy of binding values of –275 kcal mol–1 (HHb) and -253 kcal mol–1 (HbO2), represents that S1 binds with HHb more efficiently than HbO2. The sensing material S1 [(PYRH)+(DNBA)-] was characterized by SC-X-ray study, FTIR, 1H NMR, TG/DTA and Hirshfeld surface analysis. UV–vis spectrophotometry reveals that formation of S1 dependent on the solvent polarity and KCT and εCT with other physical parameters like ECT, ID, RN, ΔG, f and μEN were also calculated using spectrophotometric data. 1:1 stoichiometry of S1 was depicted by the straight-line method and the Benesi-Hildebrand equation. DFT studies provide comparable theoretical data and the HOMO-LUMO energy band gap . The N+–H---O- bonding among reactants was found to have an important role in Fe2+ interaction with S1, proving this approach of CTC synthesis as a chemosensor to be novel.
中文翻译:

基于电荷转移复合物的实时比色化学传感器,用于快速识别二硝基苯并区分检测水性介质和人血红蛋白中的Fe 2+离子
通过一步法合成了一种经济高效且易于制备的比色水介质双化学传感器(S1),其为沉淀形式的电荷转移复合物(CTC)。S1对1,3-二硝基苯显示出比其他硝基炸药高选择性的荧光猝灭响应,检出限低至0.084 ppb,结合常数为8.5110 2 M -1。S1还显示出与其他金属离子相比在Fe 2+和Fe 3+状态之间的选择性和区分性检测,这可以通过肉眼观察到Fe 2+和Fe 3+之间的色差。在人脱氧血红蛋白(HHb)和氧合血红蛋白(HbO 2)中也发现了两种状态Fe离子的判别检测,其结合常数分别为132.53 M -1和26.46 M -1。S1和血红蛋白之间的分子对接提供了–275 kcal mol –1(HHb)和-253 kcal mol –1(HbO 2)结合值的自由能)表示S1与HHb的结合比HbO 2更有效。通过SC-X射线研究,FTIR,1 H NMR,TG / DTA和Hirshfeld表面分析对传感材料S1 [(PYRH)+(DNBA)- ]进行了表征。UV-Vis分光光度法揭示了依赖于溶剂的极性和K其形成S1的CT和ε CT与喜欢E其他物理参数CT,我d,R Ñ,ΔG,f和μ EN还使用分光光度数据计算。S1的化学计量比为1:1,采用直线法和Benesi-Hildebrand方程描述。DFT研究提供了可比的理论数据和HOMO-LUMO能带隙。所述N个+ -H - O -反应物中键合被发现具有以Fe重要作用2+与S1相互作用,证明CTC合成的此方法作为化学传感器是新颖。



























 京公网安备 11010802027423号
京公网安备 11010802027423号