当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functionalization of remote C(sp3)-H bonds enabled by copper-catalyzed coupling of O-acyloximes with terminal alkynes.
Nature Communications ( IF 16.6 ) Pub Date : 2020-01-21 , DOI: 10.1038/s41467-020-14292-2 Zhaodong Li 1 , Rubén O Torres-Ochoa 2 , Qian Wang 2 , Jieping Zhu 2
Nature Communications ( IF 16.6 ) Pub Date : 2020-01-21 , DOI: 10.1038/s41467-020-14292-2 Zhaodong Li 1 , Rubén O Torres-Ochoa 2 , Qian Wang 2 , Jieping Zhu 2
Affiliation
|
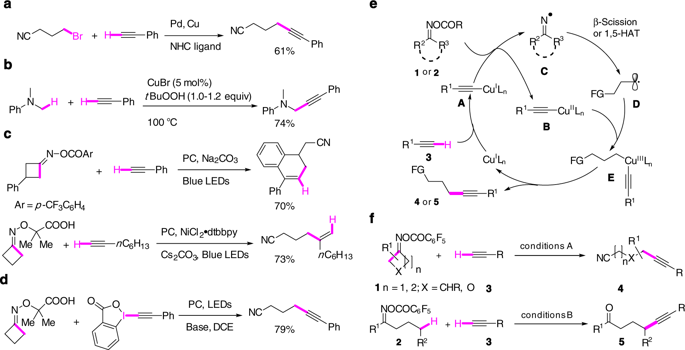
Transition metal catalyzed Sonogashira cross-coupling of terminal alkynes with aryl(vinyl) (pseudo)halides has been successfully extended to alkyl halides for the synthesis of functionalized internal alkynes. The direct alkynylation of remote unfunctionalized sp3 carbon by terminal alkynes remains difficult to realize. We report herein an approach to this synthetic challenge by developing two catalytic remote sp3 carbon alkynylation protocols. In the presence of a catalytic amount of Cu(I) salt and a tridentate ligand (tBu3-terpyridine), O-acyloximes derived from cycloalkanones and acyclic ketones are efficiently coupled with terminal alkynes to afford a variety of γ- and δ-alkynyl nitriles and γ-alkynyl ketones, respectively. These reactions proceed through a domino sequence involving copper-catalyzed reductive generation of iminyl radical followed by radical translocation via either β-scission or 1,5-hydrogen atom transfer (1,5-HAT) and copper-catalyzed alkynylation of the resulting translocated carbon radicals. The protocols are applicable to complex natural products.
中文翻译:

通过铜催化的O-酰基肟与末端炔烃的耦合,使远程C(sp3)-H键实现功能化。
过渡金属催化的末端炔烃与芳基(乙烯基)(假)卤化物的Sonogashira交叉偶联已成功地扩展至卤代烷烃,以合成功能化的内部炔烃。仍然难以实现末端炔烃对远程未官能化sp3碳的直接烷基化作用。我们在此报告了通过开发两个催化远程sp3碳炔基化方案来解决这一合成挑战的方法。在催化量的Cu(I)盐和三齿配体(tBu3-terpyridine)存在下,将衍生自环烷酮和无环酮的O-酰基肟与末端炔烃有效偶联,以提供各种γ-和δ-炔基腈和γ-炔基酮。这些反应通过多米诺骨牌序列进行,涉及铜催化的亚胺基自由基的还原生成,然后通过β断裂或1,5-氢原子转移(1,5-HAT)进行自由基易位,以及铜催化所得的易位碳的炔基化反应部首。该协议适用于复杂的天然产品。
更新日期:2020-01-22
中文翻译:

通过铜催化的O-酰基肟与末端炔烃的耦合,使远程C(sp3)-H键实现功能化。
过渡金属催化的末端炔烃与芳基(乙烯基)(假)卤化物的Sonogashira交叉偶联已成功地扩展至卤代烷烃,以合成功能化的内部炔烃。仍然难以实现末端炔烃对远程未官能化sp3碳的直接烷基化作用。我们在此报告了通过开发两个催化远程sp3碳炔基化方案来解决这一合成挑战的方法。在催化量的Cu(I)盐和三齿配体(tBu3-terpyridine)存在下,将衍生自环烷酮和无环酮的O-酰基肟与末端炔烃有效偶联,以提供各种γ-和δ-炔基腈和γ-炔基酮。这些反应通过多米诺骨牌序列进行,涉及铜催化的亚胺基自由基的还原生成,然后通过β断裂或1,5-氢原子转移(1,5-HAT)进行自由基易位,以及铜催化所得的易位碳的炔基化反应部首。该协议适用于复杂的天然产品。



























 京公网安备 11010802027423号
京公网安备 11010802027423号