当前位置:
X-MOL 学术
›
Appl. Clay. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of CuNiSn LDHs as highly efficient Fenton catalysts for degradation of phenol
Applied Clay Science ( IF 5.6 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.clay.2019.105433 Hao Wang , Zeng Zhang , Mengmeng Jing , Song Tang , Yan Wu , Wenshi Liu
Applied Clay Science ( IF 5.6 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.clay.2019.105433 Hao Wang , Zeng Zhang , Mengmeng Jing , Song Tang , Yan Wu , Wenshi Liu
|
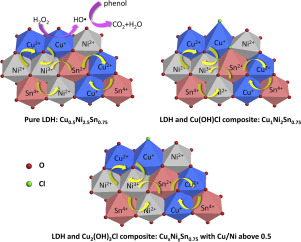
Abstract A series of CuNiSn ternary layered double hydroxides (LDH) were synthesized as catalysts for degradation of phenol via Fenton reaction. At Cu/Ni ratios above 0.2, copper chloride hydroxide co-exists with LDH. Multivalent Cu+/Cu2+, Ni2+/Ni3+ and Sn2+/Sn4+ ions are present in LDH due to the electron transfer between metals in brucite-like sheets. The catalytic activity varies directly with the Cu+ percentage, and the maximum activity is achieved at Cu/Ni/Sn ratio of 1/2/0.75. This catalyst (Cu1Ni2Sn0.75) can mineralize 97.8% phenol under mild conditions and shows good stability. Multivalent Cu, Ni and Sn cations in LDH could construct a closed electron cycle. This allows the rapid regeneration of Cu+ active species through electron transfer within LDH rather than through slow Fenton-like reaction, as what usually happens in conventional Fenton catalysts. In addition, a positive synergistic effect between copper chloride hydroxide and LDH is observed in Cu1Ni2Sn0.75, favoring the formation of Cu+.
中文翻译:

CuNiSn LDHs 的合成作为高效芬顿催化剂降解苯酚
摘要 合成了一系列CuNiSn 三元层状双氢氧化物(LDH) 作为Fenton 反应降解苯酚的催化剂。当 Cu/Ni 比率高于 0.2 时,氢氧化氯化铜与 LDH 共存。由于类水镁石片中金属之间的电子转移,LDH 中存在多价 Cu+/Cu2+、Ni2+/Ni3+ 和 Sn2+/Sn4+ 离子。催化活性直接随 Cu+ 百分比变化,在 Cu/Ni/Sn 比为 1/2/0.75 时达到最大活性。这种催化剂(Cu1Ni2Sn0.75)可以在温和条件下矿化97.8%的苯酚,并表现出良好的稳定性。LDH中的多价Cu、Ni和Sn阳离子可以构建闭合电子循环。这允许通过 LDH 内的电子转移而不是通过缓慢的类芬顿反应快速再生 Cu+ 活性物质,就像在传统的芬顿催化剂中通常发生的那样。此外,在 Cu1Ni2Sn0.75 中观察到氯化铜氢氧化物和 LDH 之间的正协同效应,有利于形成 Cu+。
更新日期:2020-03-01
中文翻译:

CuNiSn LDHs 的合成作为高效芬顿催化剂降解苯酚
摘要 合成了一系列CuNiSn 三元层状双氢氧化物(LDH) 作为Fenton 反应降解苯酚的催化剂。当 Cu/Ni 比率高于 0.2 时,氢氧化氯化铜与 LDH 共存。由于类水镁石片中金属之间的电子转移,LDH 中存在多价 Cu+/Cu2+、Ni2+/Ni3+ 和 Sn2+/Sn4+ 离子。催化活性直接随 Cu+ 百分比变化,在 Cu/Ni/Sn 比为 1/2/0.75 时达到最大活性。这种催化剂(Cu1Ni2Sn0.75)可以在温和条件下矿化97.8%的苯酚,并表现出良好的稳定性。LDH中的多价Cu、Ni和Sn阳离子可以构建闭合电子循环。这允许通过 LDH 内的电子转移而不是通过缓慢的类芬顿反应快速再生 Cu+ 活性物质,就像在传统的芬顿催化剂中通常发生的那样。此外,在 Cu1Ni2Sn0.75 中观察到氯化铜氢氧化物和 LDH 之间的正协同效应,有利于形成 Cu+。



























 京公网安备 11010802027423号
京公网安备 11010802027423号