当前位置:
X-MOL 学术
›
Appl. Clay. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption of humic acid from aqueous solution by magnetic Zn/Al calcined layered double hydroxides
Applied Clay Science ( IF 5.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.clay.2019.105414 Shaoxiu Li , Yuhe Yang , Su Huang , Zhifeng He , Changhui Li , Dongmei Li , Bohan Ke , Chan Lai , Qiuyi Peng
Applied Clay Science ( IF 5.6 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.clay.2019.105414 Shaoxiu Li , Yuhe Yang , Su Huang , Zhifeng He , Changhui Li , Dongmei Li , Bohan Ke , Chan Lai , Qiuyi Peng
|
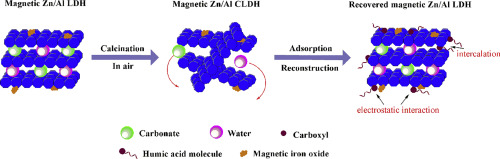
Abstract Magnetic Zn/Al layered double hydroxides (M Zn/Al LDH) were prepared by co-precipitation method and then calcined at 370 °C for 2 h to obtain magnetic Zn/Al calcined layered double hydroxides (M Zn/Al CLDH) used for adsorption of humic acid (HA) from aqueous solution. The morphology and structure of M Zn/Al CLDH were characterized by scanning electron microscope (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), specific surface area (SSA), thermogravimetric analysis and differential scanning calorimetry (TG-DSC) and vibrating-sample magnetometer (VSM), respectively. The results showed that the implant of a certain amount of magnetic substrates on Zn/Al LDH didn't damage the layered structure of Zn/Al LDH. After calcination, M Zn/Al CLDH exhibited the characteristics of zinc aluminum oxides, whose specific surface area increased to 80.00m2/g from 49.15 m2/g of M Zn/Al LDH. The magnetism of M Zn/Al LDH originating from Fe3O4 and γ-Fe2O3 kept well under calcination at 370 °C. The influence of solution pH and competitive ions on the HA removal was investigated. M Zn/Al CLDH exhibited high HA removal efficiency which was over 97% from pH 4 to pH 8 for 30 min at M Zn/Al CLDH dosage of 0.2 g/L and HA initial concentration of 20 mg/L. The kinetic, adsorption isotherm and thermodynamic of the adsorption of HA by M Zn/Al CLDH were studied. The results indicated that the adsorption followed pseudo-second-order kinetics model and Langmuir isotherm model. Thermodynamic analysis suggested that the adsorption of humic acid on M Zn/Al CLDH was a spontaneous and endothermic process. The adsorption mechanism is associated with electrostatic interaction and the intercalation of some functional groups of HA during layered structure of M Zn/Al LDH recovery in water due to the memory effect. This study revealed that M Zn/Al CLDH was an effective adsorbent for HA removal in water treatment.
中文翻译:

磁性Zn/Al煅烧层状双氢氧化物从水溶液中吸附腐植酸
摘要 采用共沉淀法制备磁性 Zn/Al 层状双氢氧化物(M Zn/Al LDH),然后在 370 °C 下煅烧 2 h,得到磁性 Zn/Al 层状双氢氧化物(M Zn/Al CLDH)。用于从水溶液中吸附腐殖酸 (HA)。通过扫描电子显微镜(SEM)、X射线衍射(XRD)、傅里叶变换红外光谱(FTIR)、比表面积(SSA)、热重分析和差示扫描量热法对M Zn/Al CLDH的形貌和结构进行了表征。 TG-DSC) 和振动样品磁强计 (VSM)。结果表明,在Zn/Al LDH上注入一定量的磁性基体并没有破坏Zn/Al LDH的层状结构。煅烧后,M Zn/Al CLDH表现出锌铝氧化物的特性,其比表面积从 M Zn/Al LDH 的 49.15 m2/g 增加到 80.00 m2/g。源自 Fe3O4 和 γ-Fe2O3 的 M Zn/Al LDH 的磁性在 370°C 煅烧下保持良好。研究了溶液 pH 值和竞争性离子对 HA 去除的影响。在 M Zn/Al CLDH 剂量为 0.2 g/L 和 HA 初始浓度为 20 mg/L 时,M Zn/Al CLDH 表现出高 HA 去除效率,从 pH 4 到 pH 8,持续 30 分钟超过 97%。研究了M Zn/Al CLDH吸附HA的动力学、吸附等温线和热力学。结果表明吸附遵循准二级动力学模型和Langmuir等温线模型。热力学分析表明,腐植酸在 M Zn/Al CLDH 上的吸附是一个自发吸热过程。由于记忆效应,M Zn / Al LDH在水中回收的层状结构期间,吸附机制与静电相互作用和HA的一些官能团的嵌入有关。该研究表明,M Zn/Al CLDH 是水处理中去除 HA 的有效吸附剂。
更新日期:2020-04-01
中文翻译:

磁性Zn/Al煅烧层状双氢氧化物从水溶液中吸附腐植酸
摘要 采用共沉淀法制备磁性 Zn/Al 层状双氢氧化物(M Zn/Al LDH),然后在 370 °C 下煅烧 2 h,得到磁性 Zn/Al 层状双氢氧化物(M Zn/Al CLDH)。用于从水溶液中吸附腐殖酸 (HA)。通过扫描电子显微镜(SEM)、X射线衍射(XRD)、傅里叶变换红外光谱(FTIR)、比表面积(SSA)、热重分析和差示扫描量热法对M Zn/Al CLDH的形貌和结构进行了表征。 TG-DSC) 和振动样品磁强计 (VSM)。结果表明,在Zn/Al LDH上注入一定量的磁性基体并没有破坏Zn/Al LDH的层状结构。煅烧后,M Zn/Al CLDH表现出锌铝氧化物的特性,其比表面积从 M Zn/Al LDH 的 49.15 m2/g 增加到 80.00 m2/g。源自 Fe3O4 和 γ-Fe2O3 的 M Zn/Al LDH 的磁性在 370°C 煅烧下保持良好。研究了溶液 pH 值和竞争性离子对 HA 去除的影响。在 M Zn/Al CLDH 剂量为 0.2 g/L 和 HA 初始浓度为 20 mg/L 时,M Zn/Al CLDH 表现出高 HA 去除效率,从 pH 4 到 pH 8,持续 30 分钟超过 97%。研究了M Zn/Al CLDH吸附HA的动力学、吸附等温线和热力学。结果表明吸附遵循准二级动力学模型和Langmuir等温线模型。热力学分析表明,腐植酸在 M Zn/Al CLDH 上的吸附是一个自发吸热过程。由于记忆效应,M Zn / Al LDH在水中回收的层状结构期间,吸附机制与静电相互作用和HA的一些官能团的嵌入有关。该研究表明,M Zn/Al CLDH 是水处理中去除 HA 的有效吸附剂。

























 京公网安备 11010802027423号
京公网安备 11010802027423号