Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-01-18 , DOI: 10.1016/j.molliq.2020.112519 Georgios Stogiannidis , Stefanos Tsigoias , Angelos G. Kalampounias
|
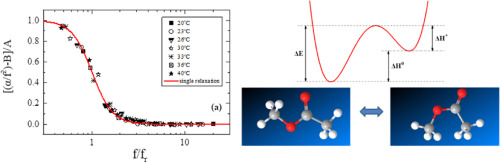
We report on a temperature-dependent ultrasonic relaxation study of the binary methyl acetate – ethanol solutions in a wide concentration and frequency range using the pulse-echo method. This system exhibits a single relaxation process assigned to a specific conformational process. The unique relaxation effect and the chemical simplicity of methyl acetate facilitate more accurate quantum mechanical calculations. From the temperature dependence of the corresponding ultrasonic relaxation parameters, we estimated the activation enthalpy (ΔH⁎ = 4.97 ± 0.42 kcal/mol) and the energy gap (ΔH0 = 5.72 ± 0.44 kcal/mol) between the cis- and trans-isomers of pure methyl acetate.
Molecular orbital calculations were performed in order to investigate the structural, spectroscopic and thermodynamic properties of both cis- and trans-conformers of methyl acetate in a vacuum environment. The trans-isomer is confirmed as the most thermodynamically stable. The Synchronous Transit-Guided Quasi-Newton (STQN) method has been used for locating the transition structures. We confirmed that a single transition structure is present and we calculated the enthalpy of this state. Subsequently, the activation enthalpy from the trans- to the cis-conformer was estimated equal to 12.42 kcal/mol. The calculated value is close to the experimentally estimated energy barrier value (ΔEexperimental = 10.69 ± 0.56 kcal/mol). In methyl acetate-ethanol solutions thermal relaxation plays a predominant role over structural relaxation process. The deviation between the experimental and calculated effective sound velocity indicates strong interactions between unlike molecules in methyl acetate-ethanol solutions analogous to that observed recently using vibrational spectroscopies.
中文翻译:

乙酸甲酯-乙醇溶液中的构象能垒:取决于温度的超声弛豫研究和分子轨道计算
我们报告了使用脉冲回波方法在宽浓度和频率范围内对二元乙酸甲酯-乙醇溶液进行温度依赖性超声弛豫研究。该系统展示了分配给特定构象过程的单个松弛过程。乙酸甲酯的独特弛豫效应和化学简单性促进了更精确的量子力学计算。由相应的超声弛豫参数的温度依赖性,我们估计活化焓(Δ ħ ⁎ = 4.97±0.42千卡/摩尔)和能隙(Δ ħ 0 = 5.72±0.44千卡/摩尔)的顺式和反之间-纯乙酸甲酯的异构体。
为了研究在真空环境中乙酸甲酯的顺式和反式构象异构体的结构,光谱和热力学性质,进行了分子轨道计算。确认反式异构体是最热力学稳定的。同步运输引导拟牛顿法(STQN)已用于定位过渡结构。我们确认存在单个过渡结构,并计算了该状态的焓。随后,估计从反式到顺式构象异构体的活化焓为12.42 kcal / mol。计算值接近于实验估计的能垒值(ΔE实验 = 10.69±0.56 kcal / mol)。在乙酸甲酯-乙醇溶液中,热弛豫在结构弛豫过程中起主要作用。实验和计算的有效声速之间的偏差表明,乙酸甲酯-乙醇溶液中不同分子之间的相互作用很强,类似于最近使用振动光谱学观察到的相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号