Separation and Purification Technology ( IF 8.6 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.seppur.2020.116600 Junjun Chang , Yuping Li , Feng Duan , Chunlei Su , Yujiao Li , Hongbin Cao
|
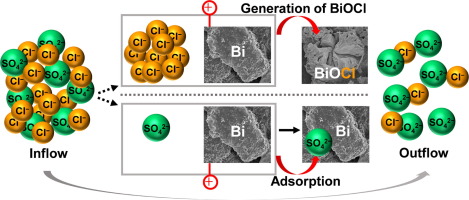
Common methods in capacitive deionization (CDI) for selective removal are derived from membrane separation mechanism or size-based intercalation mechanism. In this study, based on the electrochemical activity of bismuth (Bi) material to chloride ions (Cl−) and inertness to sulfate ions (SO42−), Bi electrode is used as anode in CDI to selectively remove Cl− ions from mixed NaCl and Na2SO4 solution. Through the reaction with Bi, Cl− ions are stored in bismuth oxychloride (BiOCl). Results show that Bi electrode exhibits selectivity for Cl− over SO42− with a selectivity coefficient of more than 1.5 in solutions with mole ratio of Cl− to SO42− larger than 1. The selectivity coefficient is greatly dependent on the mole ratio of Cl− to SO42− and increases when the ratio rises. In solution with a fixed Cl−/ SO42− mole ratio, higher applied voltage and prolonged time result in higher Cl− ion removal capacity, but not necessarily better selectivity. The highest selectivity coefficient of 4.5 is achieved in 8.5 mM NaCl and 1.1 mM Na2SO4 mixed solution at voltage of 1.6 and 2.0 V after charging for 1 h. These results demonstrate that Bi electrode can selectively remove Cl− ions from mixed solution with a relatively lower concentration of SO42− ions, which provides new insights into ion separation and selective removal by CDI.
中文翻译:

电容去离子中铋电极选择性去除氯离子
选择性去除的电容性去离子(CDI)的常用方法是从膜分离机制或基于尺寸的插层机制中衍生出来的。在这项研究中,基于铋的电化学活性(Bi)的材料,以氯离子(CL - )和惰性至硫酸根离子(SO 4 2- ),铋电极被用作阳极CDI以选择性地除去Cl -从混合离子NaCl和Na 2 SO 4溶液。通过与铋,氯反应-离子存储在氯氧化铋(的BiOCl)。结果表明,双电极,用于氯展品选择性-在SO 4 2-具有大于1.5的有Cl的摩尔比的解决方案的选择性系数-以SO 4 2-大于1的选择性系数是极大地依赖于氯的摩尔比-为SO 4 2-和当该比率上升增大。在具有固定Cl溶液- / SO 4 2-的摩尔比,在更高的氯更高的施加电压和时间的延长结果-离子清除能力,但不一定更好的选择性。在8.5 mM NaCl和1.1 mM Na 2 SO 4中达到4.5的最高选择性系数充电1小时后,混合溶液的电压为1.6和2.0V。这些结果表明,铋电极可以选择性除去Cl -从混合溶液用SO的相对较低的浓度离子4 2-离子,它提供了新的见解通过CDI离子分离和选择性去除。



























 京公网安备 11010802027423号
京公网安备 11010802027423号