Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Validation of the easyscreen flavivirus dengue alphavirus detection kit based on 3base amplification technology and its application to the 2016/17 Vanuatu dengue outbreak.
PLOS ONE ( IF 3.7 ) Pub Date : 2020-01-17 , DOI: 10.1371/journal.pone.0227550 Crystal Garae 1 , Kalkoa Kalo 1 , George Junior Pakoa 1 , Rohan Baker 2 , Phill Isaacs 2 , Douglas Spencer Millar 2
PLOS ONE ( IF 3.7 ) Pub Date : 2020-01-17 , DOI: 10.1371/journal.pone.0227550 Crystal Garae 1 , Kalkoa Kalo 1 , George Junior Pakoa 1 , Rohan Baker 2 , Phill Isaacs 2 , Douglas Spencer Millar 2
Affiliation
|
BACKGROUND
The family flaviviridae and alphaviridae contain a diverse group of pathogens that cause significant morbidity and mortality worldwide. Diagnosis of the virus responsible for disease is essential to ensure patients receive appropriate clinical management. Very few real-time RT-PCR based assays are able to detect the presence of all members of these families using a single primer and probe set. We have developed a novel chemistry, 3base, which simplifies the viral nucleic acids allowing the design of RT-PCR assays capable of pan-family identification.
METHODOLOGY/PRINCIPAL FINDING
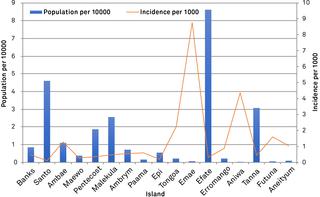
Synthetic constructs, viral nucleic acids, intact viral particles and characterised reference materials were used to determine the specificity and sensitivity of the assays. Synthetic constructs demonstrated the sensitivities of the pan-flavivirus detection component were in the range of 13 copies per PCR. The pan-alphavirus assay had a sensitivity range of 10-25 copies per reaction depending on the viral strain. Lower limit of detection studies using whole virus particles demonstrated that sensitivity for assays was in the range of 1-2 copies per reaction. No cross reactivity was observed with a number of commonly encountered viral strains. Proficiency panels showed 100% concordance with the expected results and the assays performed as well as, if not better than, other assays used in laboratories worldwide. After initial assay validation the pan-viral assays were then tested during the 2016-2017 Vanuatu dengue-2 outbreak. Positive results were detected in 116 positives from a total of 187 suspected dengue samples.
CONCLUSIONS/SIGNIFICANCE
The pan-viral screening assays described here utilise a novel 3base technology and are shown to provide a sensitive and specific method to screen and thereafter speciate flavi- and/or alpha- viruses in clinical samples. The assays performed well in an outbreak situation and can be used to detect positive clinical samples containing any flavivirus or alphavirus in approximately 3 hours 30 minutes.
中文翻译:

基于3base扩增技术的easyscreen黄病毒登革热甲病毒检测试剂盒的验证及其在2016/17瓦努阿图登革热暴发中的应用。
背景技术黄病毒科和甲病毒科包含各种各样的病原体,这些病原体在全世界范围内引起很大的发病率和死亡率。诊断引起疾病的病毒对于确保患者接受适当的临床治疗至关重要。很少有基于实时RT-PCR的检测方法能够使用单个引物和探针组检测这些家族中所有成员的存在。我们已经开发出一种新颖的化学物质3base,它可以简化病毒核酸,从而设计出能够进行泛家族鉴定的RT-PCR分析方法。方法学/主要发现使用合成的构建体,病毒核酸,完整的病毒颗粒和特征化的参考材料确定测定的特异性和敏感性。合成构建体表明,每株PCR泛黄病毒检测组分的敏感性在13个拷贝的范围内。取决于病毒株,泛甲病毒测定的每个反应灵敏度范围为10-25拷贝。使用全病毒颗粒的检测研究的下限表明,每个反应的测定灵敏度为1-2个拷贝。与许多常见的病毒株未观察到交叉反应。熟练专家小组显示出与预期结果和所执行的检测方法的一致性100%,甚至比全球实验室使用的其他检测方法更好,甚至更好。在初步分析确认后,然后在2016-2017年瓦努阿图登革热2爆发期间测试了泛病毒分析。在总共187份可疑登革热样本中,有116份阳性检测出阳性结果。结论/意义这里描述的泛病毒筛选测定法利用了一种新颖的3base技术,并被证明提供了一种灵敏而特异的方法来筛选并确定临床样品中的黄病毒和/或α病毒。该测定在暴发情况下表现良好,可用于在大约3小时30分钟内检测包含任何黄病毒或甲病毒的阳性临床样品。
更新日期:2020-01-21
中文翻译:

基于3base扩增技术的easyscreen黄病毒登革热甲病毒检测试剂盒的验证及其在2016/17瓦努阿图登革热暴发中的应用。
背景技术黄病毒科和甲病毒科包含各种各样的病原体,这些病原体在全世界范围内引起很大的发病率和死亡率。诊断引起疾病的病毒对于确保患者接受适当的临床治疗至关重要。很少有基于实时RT-PCR的检测方法能够使用单个引物和探针组检测这些家族中所有成员的存在。我们已经开发出一种新颖的化学物质3base,它可以简化病毒核酸,从而设计出能够进行泛家族鉴定的RT-PCR分析方法。方法学/主要发现使用合成的构建体,病毒核酸,完整的病毒颗粒和特征化的参考材料确定测定的特异性和敏感性。合成构建体表明,每株PCR泛黄病毒检测组分的敏感性在13个拷贝的范围内。取决于病毒株,泛甲病毒测定的每个反应灵敏度范围为10-25拷贝。使用全病毒颗粒的检测研究的下限表明,每个反应的测定灵敏度为1-2个拷贝。与许多常见的病毒株未观察到交叉反应。熟练专家小组显示出与预期结果和所执行的检测方法的一致性100%,甚至比全球实验室使用的其他检测方法更好,甚至更好。在初步分析确认后,然后在2016-2017年瓦努阿图登革热2爆发期间测试了泛病毒分析。在总共187份可疑登革热样本中,有116份阳性检测出阳性结果。结论/意义这里描述的泛病毒筛选测定法利用了一种新颖的3base技术,并被证明提供了一种灵敏而特异的方法来筛选并确定临床样品中的黄病毒和/或α病毒。该测定在暴发情况下表现良好,可用于在大约3小时30分钟内检测包含任何黄病毒或甲病毒的阳性临床样品。



























 京公网安备 11010802027423号
京公网安备 11010802027423号