Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.tetlet.2020.151645 Oleksandr O. Grygorenko
|
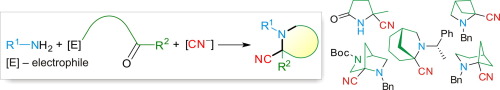
Approaches to α-cyanopyrrolidines, -piperidines, and -azepanes, as well as their bi- and polycyclic analogues are surveyed, which are based on Strecker reaction – intramolecular nucleophilic cyclization. The reactions are categorized according to the nature of the internal electrophile participating in the cyclization step, i.e. carboxylic acid or its derivative, carbonyl compound, or alkylating agent. Special attention is paid to one-pot tandem Strecker reaction – SN2-type nucleophilic cyclization (STRINC), or “cyanide-induced dynamic intramolecular cyclization”, which is an efficient and convenient approach to various mono- and bicyclic α-amino nitriles and α-amino acids.
中文翻译:

通过Strecker反应合成饱和氮杂环-亲核环化
研究了基于Strecker反应–分子内亲核环化的α-氰基吡咯烷,-哌啶和-azepanes及其双环和多环类似物的方法。根据参与环化步骤的内部亲电试剂的性质,对反应进行分类,即羧酸或其衍生物,羰基化合物或烷基化剂。特别注意一锅串联Strecker反应-S N 2型亲核环化(STRINC)或“氰化物诱导的动态分子内环化”,这是一种有效且方便的方法,用于处理各种单环和双环α-氨基腈和α-氨基酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号