Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-01-20 , DOI: 10.1016/j.tetlet.2020.151646 Sushree Ranjan Sahoo , Debayan Sarkar
|
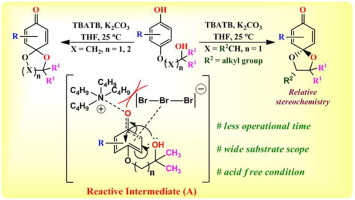
A metal free one-pot stereoselective synthesis of para-quinone monoketals and their derivatives has been reported in this present work. Tri-bromides (TBrs) have been employed as an effective reagent for intramolecular spiro-cyclizations leading to excellent yields of the monoketals. The TBrs are slowly turning out to be an important reagent for oxidative dearomatization which is further attested in the presented work. In addition, the obtained substituted para-quinone monoketals and their derivatives were readily transformed into more complex products by reaction with Pd/C under hydrogen atmosphere leading to saturated para-monoacetal, which are important building blocks in organic synthesis
中文翻译:

的立体选择性合成帕拉-quinone单缩酮通过三溴化物(TBR)的酚类化合物介导的氧化脱芳
对苯二酚单缩酮及其衍生物的无金属一锅立体选择性合成已在本工作中报道。三溴化物(TBrs)已被用作分子内螺环化的有效试剂,从而导致单缩酮的优异收率。三溴联苯逐渐被证明是氧化脱芳香化反应的重要试剂,本工作进一步证明了这一点。此外,通过在氢气气氛下与Pd / C反应,可以轻松地将获得的取代对对醌单缩酮及其衍生物转化为更复杂的产物,从而生成饱和的对一缩醛,这是有机合成的重要组成部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号