Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study.
The Lancet ( IF 168.9 ) Pub Date : 2020-01-17 , DOI: 10.1016/s0140-6736(19)33220-9 Nicholas J DeVito 1 , Seb Bacon 1 , Ben Goldacre 1
The Lancet ( IF 168.9 ) Pub Date : 2020-01-17 , DOI: 10.1016/s0140-6736(19)33220-9 Nicholas J DeVito 1 , Seb Bacon 1 , Ben Goldacre 1
Affiliation
|
BACKGROUND
Failure to report the results of a clinical trial can distort the evidence base for clinical practice, breaches researchers' ethical obligations to participants, and represents an important source of research waste. The Food and Drug Administration Amendments Act (FDAAA) of 2007 now requires sponsors of applicable trials to report their results directly onto ClinicalTrials.gov within 1 year of completion. The first trials covered by the Final Rule of this act became due to report results in January, 2018. In this cohort study, we set out to assess compliance.
METHODS
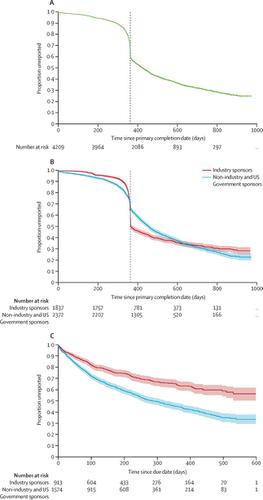
We downloaded data for all registered trials on ClinicalTrials.gov each month from March, 2018, to September, 2019. All cross-sectional analyses in this manuscript were performed on data extracted from ClinicalTrials.gov on Sept 16, 2019; monthly trends analysis used archived data closest to the 15th day of each month from March, 2018, to September, 2019. Our study cohort included all applicable trials due to report results under FDAAA. We excluded all non-applicable trials, those not yet due to report, and those given a certificate allowing for delayed reporting. A trial was considered reported if results had been submitted and were either publicly available, or undergoing quality control review at ClinicalTrials.gov. A trial was considered compliant if these results were submitted within 1 year of the primary completion date, as required by the legislation. We described compliance with the FDAAA 2007 Final Rule, assessed trial characteristics associated with results reporting using logistic regression models, described sponsor-level reporting, examined trends in reporting, and described time-to-report using the Kaplan-Meier method.
FINDINGS
4209 trials were due to report results; 1722 (40·9%; 95% CI 39·4-42·2) did so within the 1-year deadline. 2686 (63·8%; 62·4-65·3) trials had results submitted at any time. Compliance has not improved since July, 2018. Industry sponsors were significantly more likely to be compliant than non-industry, non-US Government sponsors (odds ratio [OR] 3·08 [95% CI 2·52-3·77]), and sponsors running large numbers of trials were significantly more likely to be compliant than smaller sponsors (OR 11·84 [9·36-14·99]). The median delay from primary completion date to submission date was 424 days (95% CI 412-435), 59 days higher than the legal reporting requirement of 1 year.
INTERPRETATION
Compliance with the FDAAA 2007 is poor, and not improving. To our knowledge, this is the first study to fully assess compliance with the Final Rule of the FDAAA 2007. Poor compliance is likely to reflect lack of enforcement by regulators. Effective enforcement and action from sponsors is needed; until then, open public audit of compliance for each individual sponsor may help. We will maintain updated compliance data for each individual sponsor and trial at fdaaa.trialstracker.net.
FUNDING
Laura and John Arnold Foundation.
中文翻译:

符合法律要求以在ClinicalTrials.gov上报告临床试验结果:一项队列研究。
背景技术未能报告临床试验的结果会扭曲临床实践的证据基础,违反研究人员对参与者的道德义务,并且是研究浪费的重要来源。现在,2007年《食品药品管理局修正法案》(FDAAA)要求适用试验的申办者在完成试验后的1年内直接将结果报告给ClinicalTrials.gov。该法案最终规则涵盖的第一批试验将于2018年1月发布报告结果。在此队列研究中,我们着手评估合规性。方法我们从2018年3月至2019年9月每月在ClinicalTrials.gov上下载所有已注册试验的数据。本手稿中的所有横断面分析均基于2019年9月16日从ClinicalTrials.gov上摘录的数据进行;每月趋势分析使用的数据最接近于2018年3月至2019年9月每个月的15日。由于FDAAA的报告结果,我们的研究队列包括所有适用的试验。我们排除了所有不适用的试验,尚未到期的试验以及已获得允许延迟报告的证书的试验。如果结果已经提交并且可以公开获得,或者正在ClinicalTrials.gov进行质量控制审查,则认为已报告了一项试验。如果这些结果是根据法律要求在最初完成日期的一年内提交的,则认为该试验是合规的。我们描述了是否符合FDAAA 2007最终规则,使用Logistic回归模型评估了与结果报告相关的试验特征,描述了赞助商级别的报告,检查了报告趋势,并使用Kaplan-Meier方法描述了报告时间。结果4209项试验归因于报告结果;1722(40·9%; 95%CI 39·4-42·2)在1年期限内做到了。2686(63·8%; 62·4-65·3)个试验随时都有结果提交。自2018年7月以来,合规性从未得到改善。行业赞助商比非行业,非美国政府赞助商合规的可能性要高得多(赔率[OR] 3·08 [95%CI 2·52-3·77]) ,并且进行大量试验的申办者比较小的申办者更可能合规(OR 11·84 [9·36-14·99])。从主要完成日期到提交日期的中值延迟为424天(95%CI 412-435),比1年的法律报告要求高59天。解释与FDAAA 2007的合规性很差,并且没有改善。据我们所知,这是第一项全面评估是否符合FDAAA 2007年最终规则的研究。不良的合规性可能反映出监管机构缺乏执法。需要赞助商的有效执行和采取行动;在此之前,对每个发起人进行公开的合规性公共审核可能会有帮助。我们将在fdaaa.trialstracker.net上维护每个赞助商和试验的更新合规数据。资金劳拉和约翰·阿诺德基金会。
更新日期:2020-01-31
中文翻译:

符合法律要求以在ClinicalTrials.gov上报告临床试验结果:一项队列研究。
背景技术未能报告临床试验的结果会扭曲临床实践的证据基础,违反研究人员对参与者的道德义务,并且是研究浪费的重要来源。现在,2007年《食品药品管理局修正法案》(FDAAA)要求适用试验的申办者在完成试验后的1年内直接将结果报告给ClinicalTrials.gov。该法案最终规则涵盖的第一批试验将于2018年1月发布报告结果。在此队列研究中,我们着手评估合规性。方法我们从2018年3月至2019年9月每月在ClinicalTrials.gov上下载所有已注册试验的数据。本手稿中的所有横断面分析均基于2019年9月16日从ClinicalTrials.gov上摘录的数据进行;每月趋势分析使用的数据最接近于2018年3月至2019年9月每个月的15日。由于FDAAA的报告结果,我们的研究队列包括所有适用的试验。我们排除了所有不适用的试验,尚未到期的试验以及已获得允许延迟报告的证书的试验。如果结果已经提交并且可以公开获得,或者正在ClinicalTrials.gov进行质量控制审查,则认为已报告了一项试验。如果这些结果是根据法律要求在最初完成日期的一年内提交的,则认为该试验是合规的。我们描述了是否符合FDAAA 2007最终规则,使用Logistic回归模型评估了与结果报告相关的试验特征,描述了赞助商级别的报告,检查了报告趋势,并使用Kaplan-Meier方法描述了报告时间。结果4209项试验归因于报告结果;1722(40·9%; 95%CI 39·4-42·2)在1年期限内做到了。2686(63·8%; 62·4-65·3)个试验随时都有结果提交。自2018年7月以来,合规性从未得到改善。行业赞助商比非行业,非美国政府赞助商合规的可能性要高得多(赔率[OR] 3·08 [95%CI 2·52-3·77]) ,并且进行大量试验的申办者比较小的申办者更可能合规(OR 11·84 [9·36-14·99])。从主要完成日期到提交日期的中值延迟为424天(95%CI 412-435),比1年的法律报告要求高59天。解释与FDAAA 2007的合规性很差,并且没有改善。据我们所知,这是第一项全面评估是否符合FDAAA 2007年最终规则的研究。不良的合规性可能反映出监管机构缺乏执法。需要赞助商的有效执行和采取行动;在此之前,对每个发起人进行公开的合规性公共审核可能会有帮助。我们将在fdaaa.trialstracker.net上维护每个赞助商和试验的更新合规数据。资金劳拉和约翰·阿诺德基金会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号