当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Smartphone‐Assisted Sensitive, Selective and Reversible Recognition of Copper Ions in an Aqueous Medium
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-01-20 , DOI: 10.1002/slct.201904399 Arvind Kumar 1, 2 , Anuradha Bera 3 , Satish Kumar 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-01-20 , DOI: 10.1002/slct.201904399 Arvind Kumar 1, 2 , Anuradha Bera 3 , Satish Kumar 1
Affiliation
|
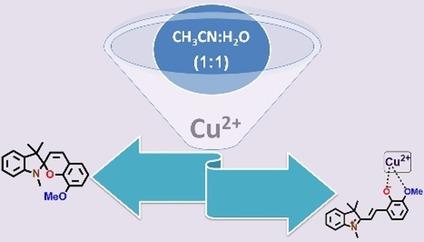
A photochromic substituted spiropyran (8‐methoxy‐1,3’,3’‐trimethylspiro[chromene‐2,2’indoline]) was successfully synthesized and its structural integrity was ascertained using spectroscopic methods. Owing to the uniquely positioned methoxy and phenolic groups for potential coordination with a metal ion, it was investigated for the recognition of toxic metal ions. The spiropyran derivative responded to the presence of Cu2+ ions in an aqueous solution by displaying a color change visible to the naked eye (colorless to pink). The color change was witnessed due to the Cu2+ ion‐induced transformation of the closed‐form (spiro) of the substituted spiropyran derivative into an open merocyanine (MC) form, which complexes the Cu2+ ion. The color change was further used for the quantification of Cu2+ ion concentration in water using a smartphone captured digital images via pixel intensity analysis. The spiropyran derivative displayed 0.24±0.01 μM, 0.65±0.06 μM (0.61±0.06 μM using paper strips) as the LOD for Cu2+ ions using UV‐Visible spectroscopy and digital colorimetry, respectively. The density functional theory (DFT) calculations and Job's plot supported the formation of a 2 : 1 (H: G) complex between the spiropyran derivative and copper ions. The time‐dependent DFT (TD‐DFT) investigations were also used to understand the color change during the complex formation, which indicated a good correlation between the experimental and theoretical results at the molecular level.
中文翻译:

智能手机辅助的水性介质中铜离子的灵敏,选择性和可逆识别
已成功合成了光致变色取代的螺吡喃(8-甲氧基-1,3',3'-三甲基螺[铬烯-2,2'二氢吲哚]),并使用光谱法确定了其结构完整性。由于与金属离子潜在配位的独特位置的甲氧基和酚基,人们对其进行了有毒金属离子的识别研究。螺吡喃衍生物通过显示肉眼可见的颜色变化(无色至粉红色)来响应水溶液中Cu 2+离子的存在。观察到颜色变化是由于Cu 2+离子诱导的取代螺吡喃衍生物的闭环形式(spiro)转变为开阔的花菁(MC)形式,从而使Cu 2+络合离子。使用智能手机通过像素强度分析捕获的数字图像,将颜色变化进一步用于量化水中的Cu 2+离子浓度。螺吡喃衍生物分别显示0.24±0.01μM,0.65±0.06μM(使用纸条为0.61±0.06μM)作为使用UV-可见光谱和数字比色法测定Cu 2+离子的LOD 。密度泛函理论(DFT)的计算和Job的图支持了螺吡喃衍生物和铜离子之间2:1(H:G)配合物的形成。随时间变化的DFT(TD-DFT)研究还用于了解复合物形成过程中的颜色变化,这表明在分子水平上的实验结果与理论结果之间具有良好的相关性。
更新日期:2020-01-21
中文翻译:

智能手机辅助的水性介质中铜离子的灵敏,选择性和可逆识别
已成功合成了光致变色取代的螺吡喃(8-甲氧基-1,3',3'-三甲基螺[铬烯-2,2'二氢吲哚]),并使用光谱法确定了其结构完整性。由于与金属离子潜在配位的独特位置的甲氧基和酚基,人们对其进行了有毒金属离子的识别研究。螺吡喃衍生物通过显示肉眼可见的颜色变化(无色至粉红色)来响应水溶液中Cu 2+离子的存在。观察到颜色变化是由于Cu 2+离子诱导的取代螺吡喃衍生物的闭环形式(spiro)转变为开阔的花菁(MC)形式,从而使Cu 2+络合离子。使用智能手机通过像素强度分析捕获的数字图像,将颜色变化进一步用于量化水中的Cu 2+离子浓度。螺吡喃衍生物分别显示0.24±0.01μM,0.65±0.06μM(使用纸条为0.61±0.06μM)作为使用UV-可见光谱和数字比色法测定Cu 2+离子的LOD 。密度泛函理论(DFT)的计算和Job的图支持了螺吡喃衍生物和铜离子之间2:1(H:G)配合物的形成。随时间变化的DFT(TD-DFT)研究还用于了解复合物形成过程中的颜色变化,这表明在分子水平上的实验结果与理论结果之间具有良好的相关性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号