当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
α-Aminoisobutyric Acid-Stabilized Peptide SAMs with Low Nonspecific Protein Adsorption and Resistance against Marine Biofouling
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-02-10 , DOI: 10.1021/acssuschemeng.9b05889 Cindy D. Beyer , Matthew L. Reback , Srinivasa M. Gopal , Kim A. Nolte , John A. Finlay 1 , Anthony S. Clare 1 , Lars V. Schäfer , Nils Metzler-Nolte , Axel Rosenhahn
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-02-10 , DOI: 10.1021/acssuschemeng.9b05889 Cindy D. Beyer , Matthew L. Reback , Srinivasa M. Gopal , Kim A. Nolte , John A. Finlay 1 , Anthony S. Clare 1 , Lars V. Schäfer , Nils Metzler-Nolte , Axel Rosenhahn
Affiliation
|
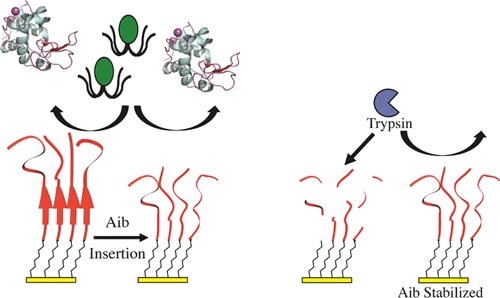
A series of low fouling peptide self-assembled monolayers (SAMs) was developed to understand how the effects of subtle sequence alterations determine the properties of peptide-terminated SAMs and settlement and adhesion of two model fouling organisms, the green alga Ulva linza and the diatom Navicula perminuta, and adsorption of two different proteins, fibrinogen and lysozyme. Insertion of the bulky, nonproteinogenic amino acid α-aminoisobutyric acid (Aib) was examined for how it affects the peptide surfaces and performance in the assays. By exchanging the serine (S) of the sequence (SGKGSSGSS) with alanine (A), we slightly altered the hydrophilicity and found reduced fouling by N. perminuta. The inclusion of Aib residues resulted in surface structural changes of the peptides from a mixture of β-sheet/random coil to strictly random coil and a decrease in the overall packing density by about 17–37%. Notably, these changes had little effect on the ability of the surface to resist nonspecific adsorption of fibrinogen and lysozyme and attachment of N. perminuta. The sequences containing Aib were 50–84% better than without Aib against the settlement of the zoospore of U. linza. Furthermore, the inclusion of Aib helped to create peptides that were 100% resistant against enzymatic degradation by trypsin, whereas the peptides without Aib were 95% degraded after 4 h.
中文翻译:

非特异性蛋白质吸附低且抗海洋生物污染的α-氨基异丁酸稳定的肽SAM
开发了一系列低污染的多肽自组装单分子膜(SAMs),以了解微妙的序列改变的影响如何确定肽终止的SAMs的特性以及两种模式污染生物(绿藻Ulva linza和硅藻)的沉降和粘附Navicula perminuta,以及两种不同蛋白,纤维蛋白原和溶菌酶的吸附。检查了大块的非蛋白原氨基酸α-氨基异丁酸(Aib)的插入物,它如何影响肽表面和检测性能。通过将序列(SGKGSSGSS)的丝氨酸(S)与丙氨酸(A)交换,我们略微改变了亲水性并发现减少了Perminuta结垢。包含Aib残基会导致肽的表面结构发生变化,从β-折叠/无规卷曲混合物变为严格随机卷曲,并使总堆积密度降低约17-37%。值得注意的是,这些变化对表面抵抗纤维蛋白原和溶菌酶的非特异性吸附以及对猪笼草的附着的能力影响很小。含有Aib的序列比U. linza游动孢子的沉降要好50-84%。此外,包含Aib有助于创建对胰蛋白酶对酶促降解具有100%抗性的肽,而不含Aib的肽在4 h后被降解95%。
更新日期:2020-02-10
中文翻译:

非特异性蛋白质吸附低且抗海洋生物污染的α-氨基异丁酸稳定的肽SAM
开发了一系列低污染的多肽自组装单分子膜(SAMs),以了解微妙的序列改变的影响如何确定肽终止的SAMs的特性以及两种模式污染生物(绿藻Ulva linza和硅藻)的沉降和粘附Navicula perminuta,以及两种不同蛋白,纤维蛋白原和溶菌酶的吸附。检查了大块的非蛋白原氨基酸α-氨基异丁酸(Aib)的插入物,它如何影响肽表面和检测性能。通过将序列(SGKGSSGSS)的丝氨酸(S)与丙氨酸(A)交换,我们略微改变了亲水性并发现减少了Perminuta结垢。包含Aib残基会导致肽的表面结构发生变化,从β-折叠/无规卷曲混合物变为严格随机卷曲,并使总堆积密度降低约17-37%。值得注意的是,这些变化对表面抵抗纤维蛋白原和溶菌酶的非特异性吸附以及对猪笼草的附着的能力影响很小。含有Aib的序列比U. linza游动孢子的沉降要好50-84%。此外,包含Aib有助于创建对胰蛋白酶对酶促降解具有100%抗性的肽,而不含Aib的肽在4 h后被降解95%。


























 京公网安备 11010802027423号
京公网安备 11010802027423号