当前位置:
X-MOL 学术
›
BBA Gen. Subj.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The lac repressor hinge helix in context: The effect of the DNA binding domain and symmetry.
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.bbagen.2020.129538 Danielle Seckfort 1 , Gillian C Lynch 2 , B Montgomery Pettitt 1
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.bbagen.2020.129538 Danielle Seckfort 1 , Gillian C Lynch 2 , B Montgomery Pettitt 1
Affiliation
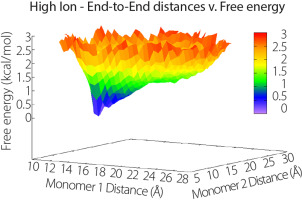
|
The Lac system of genes has been an important model system in understanding gene regulation. When the dimer lac repressor protein binds to the correct DNA sequence, the hinge region of the protein goes through a disorder to order transition. The hinge region is disordered when binding to nonoperator sequences. This region of the protein must pay a conformational entropic penalty to order when it is bound to operator DNA. Structural studies show that this region is flexible. Previous simulations showed that this region is disordered when free in solution without the DNA binding domain present. Our simulations corroborate that this region is extremely flexible in solution, but we find that the presence of the DNA binding domain proximal to the hinge helix and salt make the ordered conformation more favorable even without DNA present.
中文翻译:

lac阻遏物铰链螺旋在上下文中:DNA结合结构域和对称性的作用。
基因的Lac系统已经成为理解基因调控的重要模型系统。当二聚体lac阻遏蛋白与正确的DNA序列结合时,该蛋白的铰链区会经历无序排列的过渡。当与非操纵序列结合时,铰链区是无序的。当与操作员DNA结合时,该蛋白质的这一区域必须付出一定的构象熵损失来命令。结构研究表明,该区域是灵活的。先前的模拟表明,该区域在无DNA结合结构域的溶液中自由时是无序的。我们的模拟证实了该区域在溶液中非常灵活,但是我们发现,即使不存在DNA,在铰链螺旋和盐附近的DNA结合结构域的存在也使有序构象更加有利。
更新日期:2020-01-17
中文翻译:

lac阻遏物铰链螺旋在上下文中:DNA结合结构域和对称性的作用。
基因的Lac系统已经成为理解基因调控的重要模型系统。当二聚体lac阻遏蛋白与正确的DNA序列结合时,该蛋白的铰链区会经历无序排列的过渡。当与非操纵序列结合时,铰链区是无序的。当与操作员DNA结合时,该蛋白质的这一区域必须付出一定的构象熵损失来命令。结构研究表明,该区域是灵活的。先前的模拟表明,该区域在无DNA结合结构域的溶液中自由时是无序的。我们的模拟证实了该区域在溶液中非常灵活,但是我们发现,即使不存在DNA,在铰链螺旋和盐附近的DNA结合结构域的存在也使有序构象更加有利。


























 京公网安备 11010802027423号
京公网安备 11010802027423号