Chem ( IF 23.5 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.chempr.2019.12.021 Jian Zhang , Ziyun Wang , Wenxing Chen , Yu Xiong , Weng-Chon Cheong , Lirong Zheng , Wensheng Yan , Lin Gu , Chen Chen , Qing Peng , P. Hu , Dingsheng Wang , Yadong Li
|
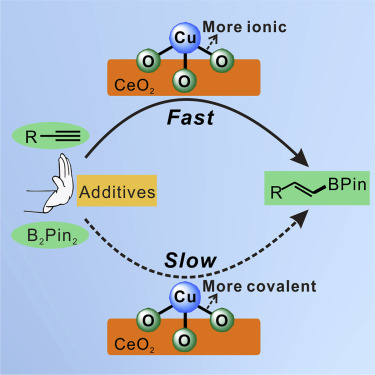
The concept of chemical bond polarity is well recognized; however, its tunability and corresponding effects on heterogeneous catalysis have never been discussed. Here, we report a method to control the polarity of the Cu-O bond in the heterogeneous Cu catalyst, and thus tune its activity for the hydroboration reaction. By synthetic procedure control, single-atomic-site Cu catalysts on ceria with more ionic Cu-O bonds (Cu1-O(I)/CeO2) and with more covalent Cu-O bonds (Cu1-O(C)/CeO2) can be obtained, respectively. The more ionic Cu-O bond makes Cu1-O(I)/CeO2 display a much higher activity than Cu1-O(C)/CeO2 in selective hydroboration of diverse alkynes with no additives, producing versatile vinylboronate compounds. The enhanced activity stems from the more ionic Cu-O bond facilitating the formation of key intermediate copper ethoxide species by dissociating ethanol molecules. These results may improve our understanding of the correlation between the nature of chemical bonds and catalytic properties and lead to better-performing heterogeneous catalysts.
中文翻译:

调整多相铜催化剂中Cu-O键的极性以促进炔烃的无添加剂加氢硼化
化学键极性的概念已得到公认。然而,从未讨论过其可调谐性及其对多相催化的影响。在这里,我们报告了一种控制非均相Cu催化剂中Cu-O键极性的方法,从而调节了其在硼氢化反应中的活性。通过合成程序控制,二氧化铈上具有更多离子性Cu-O键(Cu 1 -O(I)/ CeO 2)和共价Cu-O键(Cu 1 -O(C)/可以分别获得CeO 2)。更具离子性的Cu-O键使Cu 1 -O(I)/ CeO 2的活性大大高于Cu 1 -O(C)/ CeO 2的活性在没有添加剂的情况下,对各种炔烃进行选择性硼氢化,从而生产出多用途的乙烯基硼酸酯化合物。增强的活性源于更具离子性的Cu-O键,可通过解离乙醇分子促进关键的中间乙醇铜物种的形成。这些结果可能会增进我们对化学键性质与催化性能之间关系的理解,并导致性能更好的多相催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号