Tetrahedron ( IF 2.1 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.tet.2020.130952 Monalisa Kundu , Arin Gucchait , Anup Kumar Misra
|
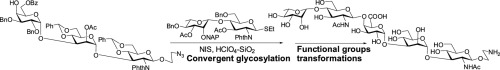
A pentasaccharide corresponding to the cell wall O-polysaccharide of the enteropathogenic Escherichia coli O115 has been synthesized using a convergent block glycosylation strategy in satisfactory yield. The synthetic strategy involves several stereoselective glycosylation steps, in which a remote picolinoyl group at C-3 mediated hydrogen bond acceptor aglycon delivery approach for the beta-linked L-rhamnosylation is worth mentioning. An orthogonal glycosylation approach has also been adopted for the preparation of a thioglycoside disaccharide derivative, which was used in the block synthesis. Perchloric acid supported over silica (HClO4–SiO2) has been used as a solid acid catalyst for the activation of glycosyl trichloroacetimidate derivative as well as in the thioglycoside activations. The d-galacturonic acid moiety in the molecules was incorporated by linking a d-galactose unit in appropriate glycosyl linkage followed by a late stage TEMPO mediated regioselective oxidation of the primary hydroxyl group using diacetoxyiodo benzene (DAIB). All intermediate glycosylations were high yielding with satisfactory stereo outcome.
中文翻译:

聚合合成对应于肠致病性大肠杆菌O115细胞壁O多糖的五糖
已经使用收敛性嵌段糖基化策略以令人满意的产率合成了对应于肠致病性大肠杆菌O115的细胞壁O-多糖的五糖。合成策略涉及几个立体选择性糖基化步骤,其中值得一提的是C-3介导的氢键受体糖苷配基在β-3连接的L-鼠李糖基化反应中的远程吡啶啉基。正交糖基化方法也已经被用于制备硫代糖苷二糖衍生物,其被用于嵌段合成中。硅石上负载的高氯酸(HClO 4 -SiO 2)已被用作固体酸催化剂用于糖基三氯乙亚氨酸酯衍生物的活化以及硫糖苷的活化。的d在分子-galacturonic酸部分通过连接方式并入d在适当的糖基连接,随后后期TEMPO半乳糖单元介导的使用二乙酰氧基碘苯(DAIB)的伯羟基的区域选择性氧化。所有中间糖基化都是高产率的,具有令人满意的立体结果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号