当前位置:
X-MOL 学术
›
J. Mol. Cell. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Osteoprotegerin promotes intimal hyperplasia and contributes to in-stent restenosis: Role of an αVβ3/FAK dependent YAP pathway.
Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.yjmcc.2020.01.006 Yuhu He 1 , Pu Zou 2 , Yufei Lu 3 , Daile Jia 4 , Xuping Li 2 , Hui Yang 2 , Liang Tang 2 , Zhaowei Zhu 2 , Tao Tu 2 , Shi Tai 2 , Yichao Xiao 2 , Mingxian Chen 2 , Lin Lu 4 , Shenghua Zhou 2
Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.yjmcc.2020.01.006 Yuhu He 1 , Pu Zou 2 , Yufei Lu 3 , Daile Jia 4 , Xuping Li 2 , Hui Yang 2 , Liang Tang 2 , Zhaowei Zhu 2 , Tao Tu 2 , Shi Tai 2 , Yichao Xiao 2 , Mingxian Chen 2 , Lin Lu 4 , Shenghua Zhou 2
Affiliation
|
OBJECTIVE
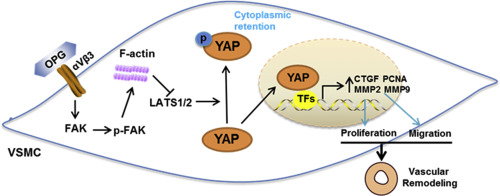
Abnormal proliferation and migration of vascular smooth muscle cells (VSMCs) are related to in-stent-restenosis (ISR) following percutaneous coronary intervention (PCI). Osteoprotegerin (OPG) has been implicated in various vascular diseases. However, the effects of OPG on ISR and the underlying mechanism remained elusive. We here investigated the association between OPG and ISR, and to demonstrate the role and potential mechanisms of OPG in neointimal hyperplasia.
APPROACH AND RESULTS
From 2962 patients who received coronary angiography and follow-up coronary angiography at approximately one year, 291 patients were diagnosed with ISR, and another 291 gender- and age- matched patients without ISR were selected as controls. Serum OPG levels were significantly increased in patients with ISR. Multivariable logistic regression analysis indicated that OPG level was independently associated with the increased risk of ISR. In a mouse femoral artery wire injury model, upregulated OPG was evidenced in vascular tissue after injury. OPG deletion attenuated the vascular injury-induced neointimal hyperplasia and related gene expression in mice. OPG promoted neointimal hyperplasia and human aortic smooth muscle cell (hASMC) proliferation and migration through activation of yes-associated protein (YAP), a major downstream effector of the Hippo signaling pathway, whereas knockdown or inhibition of YAP in hASMCs blunted OPG-induced above effects. Moreover, we found that OPG, as a ligand for integrin αVβ3, mediated phosphorylation of focal adhesion kinase (FAK) and actin cytoskeleton reorganization, resulting in YAP dephosphorylation in hASMCs. OPG-dependent YAP and VSMC activation was prevented by treatment with αVβ3-blocking antibodies and inhibitors of FAK and actin stress fibers.
CONCLUSIONS
Increased serum OPG levels are associated with increased risk of ISR following PCI and OPG could promote neointimal hyperplasia in response to injury through integrin αVβ3 mediated FAK and YAP activation, indicating OPG/YAP inhibition might serve as an attractive novel target for the prevention of ISR after PCI.
中文翻译:

骨保护素促进内膜增生并促进支架内再狭窄:αVβ3/ FAK依赖性YAP途径的作用。
目的经皮冠状动脉介入治疗(PCI)后,血管平滑肌细胞(VSMC)的异常增殖和迁移与支架内再狭窄(ISR)有关。骨保护素(OPG)已涉及多种血管疾病。但是,OPG对ISR及其基本机制的影响仍然难以捉摸。我们在这里调查了OPG和ISR之间的关联,并证明了OPG在新内膜增生中的作用和潜在机制。方法和结果从2962例接受了大约1年的冠状动脉造影和随访冠状动脉造影检查的患者中,诊断出291例患有ISR的患者,另选择291例性别和年龄相匹配的无ISR的患者作为对照。ISR患者的血清OPG水平显着增加。多变量logistic回归分析表明,OPG水平与ISR风险增加独立相关。在小鼠股动脉钢丝损伤模型中,损伤后血管组织中的OPG明显升高。OPG缺失可减轻小鼠血管损伤引起的内膜增生及相关基因的表达。OPG通过激活河马信号通路的主要下游效应因子相关蛋白(YAP)促进新内膜增生和人主动脉平滑肌细胞(hASMC)增殖和迁移,而敲除或抑制hASMC中的YAP抑制了上述OPG诱导效果。此外,我们发现OPG作为整联蛋白αVβ3的配体介导了粘着斑激酶(FAK)的磷酸化和肌动蛋白细胞骨架的重组,从而导致hASMC中的YAP去磷酸化。通过使用αVβ3阻断抗体以及FAK和肌动蛋白应激纤维抑制剂可以防止OPG依赖性YAP和VSMC活化。结论PCI后血清OPG水平升高与ISR风险增加有关,OPG可通过整合素αVβ3介导的FAK和YAP活化促进内膜增生,提示OPG / YAP抑制可能是预防ISR的一个有吸引力的新靶标在PCI之后。
更新日期:2020-01-17
中文翻译:

骨保护素促进内膜增生并促进支架内再狭窄:αVβ3/ FAK依赖性YAP途径的作用。
目的经皮冠状动脉介入治疗(PCI)后,血管平滑肌细胞(VSMC)的异常增殖和迁移与支架内再狭窄(ISR)有关。骨保护素(OPG)已涉及多种血管疾病。但是,OPG对ISR及其基本机制的影响仍然难以捉摸。我们在这里调查了OPG和ISR之间的关联,并证明了OPG在新内膜增生中的作用和潜在机制。方法和结果从2962例接受了大约1年的冠状动脉造影和随访冠状动脉造影检查的患者中,诊断出291例患有ISR的患者,另选择291例性别和年龄相匹配的无ISR的患者作为对照。ISR患者的血清OPG水平显着增加。多变量logistic回归分析表明,OPG水平与ISR风险增加独立相关。在小鼠股动脉钢丝损伤模型中,损伤后血管组织中的OPG明显升高。OPG缺失可减轻小鼠血管损伤引起的内膜增生及相关基因的表达。OPG通过激活河马信号通路的主要下游效应因子相关蛋白(YAP)促进新内膜增生和人主动脉平滑肌细胞(hASMC)增殖和迁移,而敲除或抑制hASMC中的YAP抑制了上述OPG诱导效果。此外,我们发现OPG作为整联蛋白αVβ3的配体介导了粘着斑激酶(FAK)的磷酸化和肌动蛋白细胞骨架的重组,从而导致hASMC中的YAP去磷酸化。通过使用αVβ3阻断抗体以及FAK和肌动蛋白应激纤维抑制剂可以防止OPG依赖性YAP和VSMC活化。结论PCI后血清OPG水平升高与ISR风险增加有关,OPG可通过整合素αVβ3介导的FAK和YAP活化促进内膜增生,提示OPG / YAP抑制可能是预防ISR的一个有吸引力的新靶标在PCI之后。



























 京公网安备 11010802027423号
京公网安备 11010802027423号