当前位置:
X-MOL 学术
›
Pharmacogenomics J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pharmacogenomic associations of adverse drug reactions in asthma: systematic review and research prioritisation.
The Pharmacogenomics Journal ( IF 2.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41397-019-0140-y Charlotte King 1 , Amanda McKenna 1 , Niloufar Farzan 2 , Susanne J Vijverberg 2 , Marc P van der Schee 2 , Anke H Maitland-van der Zee 2 , Lambang Arianto 3 , Hans Bisgaard 3 , Klaus BØnnelykke 3 , Vojko Berce 4, 5 , Uros PotoČnik 5 , Katja Repnik 5 , Bruce Carleton 6 , Denise Daley 6 , Fook Tim Chew 7, 8 , Wen Chin Chiang 7, 8 , Yang Yie Sio 7, 8 , Michelle M Cloutier 9 , Herman T Den Dekker 10 , Liesbeth Duijts 10 , Johan C de Jongste 11 , F Nicole Dijk 11, 12 , Carlos Flores 13, 14, 15 , Natalia Hernandez-Pacheco 13, 16 , Somnath Mukhopadhyay 17 , Kaninika Basu 17 , Kelan G Tantisira 18, 19 , Katia M Verhamme 20 , Juan C Celedón 21 , Erick Forno 21 , Glorisa Canino 22 , Ben Francis 23 , Munir Pirmohamed 24 , Ian Sinha 25 , Daniel B Hawcutt 1, 26
The Pharmacogenomics Journal ( IF 2.8 ) Pub Date : 2020-01-17 , DOI: 10.1038/s41397-019-0140-y Charlotte King 1 , Amanda McKenna 1 , Niloufar Farzan 2 , Susanne J Vijverberg 2 , Marc P van der Schee 2 , Anke H Maitland-van der Zee 2 , Lambang Arianto 3 , Hans Bisgaard 3 , Klaus BØnnelykke 3 , Vojko Berce 4, 5 , Uros PotoČnik 5 , Katja Repnik 5 , Bruce Carleton 6 , Denise Daley 6 , Fook Tim Chew 7, 8 , Wen Chin Chiang 7, 8 , Yang Yie Sio 7, 8 , Michelle M Cloutier 9 , Herman T Den Dekker 10 , Liesbeth Duijts 10 , Johan C de Jongste 11 , F Nicole Dijk 11, 12 , Carlos Flores 13, 14, 15 , Natalia Hernandez-Pacheco 13, 16 , Somnath Mukhopadhyay 17 , Kaninika Basu 17 , Kelan G Tantisira 18, 19 , Katia M Verhamme 20 , Juan C Celedón 21 , Erick Forno 21 , Glorisa Canino 22 , Ben Francis 23 , Munir Pirmohamed 24 , Ian Sinha 25 , Daniel B Hawcutt 1, 26
Affiliation
|
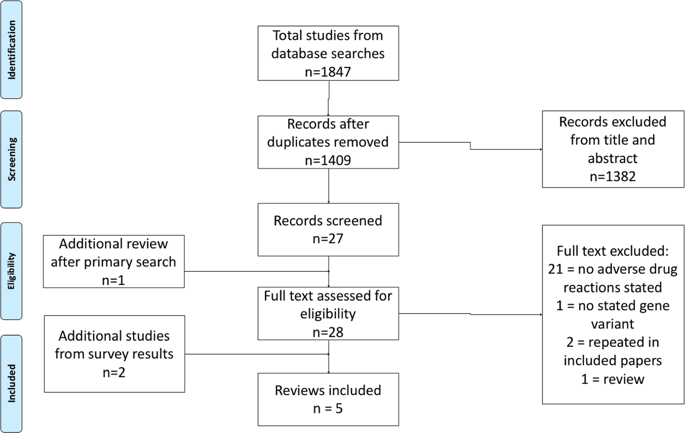
A systematic review of pharmacogenomic studies capturing adverse drug reactions (ADRs) related to asthma medications was undertaken, and a survey of Pharmacogenomics in Childhood Asthma (PiCA) consortia members was conducted. Studies were eligible if genetic polymorphisms were compared with suspected ADR(s) in a patient with asthma, as either a primary or secondary outcome. Five studies met the inclusion criteria. The ADRs and polymorphisms identified were change in lung function tests (rs1042713), adrenal suppression (rs591118), and decreased bone mineral density (rs6461639) and accretion (rs9896933, rs2074439). Two of these polymorphisms were replicated within the paper, but none had external replication. Priorities from PiCA consortia members (representing 15 institution in eight countries) for future studies were tachycardia (SABA/LABA), adrenal suppression/crisis and growth suppression (corticosteroids), sleep/behaviour disturbances (leukotriene receptor antagonists), and nausea and vomiting (theophylline). Future pharmacogenomic studies in asthma should collect relevant ADR data as well as markers of efficacy.
中文翻译:

哮喘中药物不良反应的药物基因组学关联:系统评价和研究优先次序。
对捕获与哮喘药物相关的不良药物反应(ADR)的药物基因组学研究进行了系统的综述,并对儿童哮喘(PiCA)成员的药物基因组学进行了调查。如果将遗传多态性与哮喘患者的可疑ADR进行比较,则该研究是合格的,无论是主要结果还是次要结果。五项研究符合纳入标准。在肺功能测试(rs1042713),肾上腺抑制(rs591118)以及骨矿物质密度(rs6461639)和增生(rs9896933,rs2074439)降低的情况下,发现的ADR和多态性发生了变化。这些多态性中的两个在论文中被复制,但是没有一个具有外部复制。PiCA联盟成员(代表八个国家的15个机构)的未来研究重点是心动过速(SABA / LABA),肾上腺抑制/危机和生长抑制(皮质类固醇),睡眠/行为障碍(白三烯受体拮抗剂)以及恶心和呕吐(茶碱)。未来有关哮喘的药物基因组学研究应收集相关的ADR数据以及疗效指标。
更新日期:2020-01-17
中文翻译:

哮喘中药物不良反应的药物基因组学关联:系统评价和研究优先次序。
对捕获与哮喘药物相关的不良药物反应(ADR)的药物基因组学研究进行了系统的综述,并对儿童哮喘(PiCA)成员的药物基因组学进行了调查。如果将遗传多态性与哮喘患者的可疑ADR进行比较,则该研究是合格的,无论是主要结果还是次要结果。五项研究符合纳入标准。在肺功能测试(rs1042713),肾上腺抑制(rs591118)以及骨矿物质密度(rs6461639)和增生(rs9896933,rs2074439)降低的情况下,发现的ADR和多态性发生了变化。这些多态性中的两个在论文中被复制,但是没有一个具有外部复制。PiCA联盟成员(代表八个国家的15个机构)的未来研究重点是心动过速(SABA / LABA),肾上腺抑制/危机和生长抑制(皮质类固醇),睡眠/行为障碍(白三烯受体拮抗剂)以及恶心和呕吐(茶碱)。未来有关哮喘的药物基因组学研究应收集相关的ADR数据以及疗效指标。


























 京公网安备 11010802027423号
京公网安备 11010802027423号