当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conversion of an Injectable MMP-Degradable Hydrogel into Core-Cross-Linked Micelles.
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.biomac.9b01675 Marzieh Najafi 1 , Hamed Asadi 1, 2 , Joep van den Dikkenberg 1 , Mies J van Steenbergen 1 , Marcel H A M Fens 1 , Wim E Hennink 1 , Tina Vermonden 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-05-11 , DOI: 10.1021/acs.biomac.9b01675 Marzieh Najafi 1 , Hamed Asadi 1, 2 , Joep van den Dikkenberg 1 , Mies J van Steenbergen 1 , Marcel H A M Fens 1 , Wim E Hennink 1 , Tina Vermonden 1
Affiliation
|
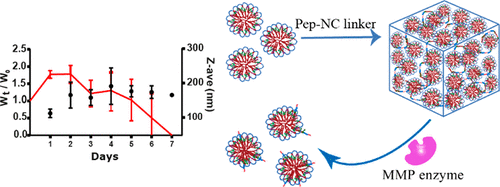
In this study, a new type of injectable hydrogel called "HyMic" that can convert into core cross-linked (CCL) micelles upon exposure to matrix metalloproteinases (MMP's), was designed and developed for drug delivery applications. HyMic is composed of CCL micelles connected via an enzyme cleavable linker. To this end, two complementary ABA block copolymers with polyethylene glycol (PEG) as B block were synthesized using atom transfer radical polymerization (ATRP). The A blocks were composed of a random copolymer of N-isopropylacrylamide (NIPAM) and either N-(2-hydroxypropyl)methacrylamide-cysteine (HPMA-Cys) or N-(2-hydroxypropyl) methacrylamide-ethylthioglycolate succinic acid (HPMA-ETSA). Mixing the aqueous solutions of the obtained polymers and rising the temperature above the cloud point of the PNIPAM block resulted in the self-assembly of these polymers into flower-like micelles composed of a hydrophilic PEG shell and hydrophobic core. The micellar core was cross-linked by native chemical ligation between the cysteine (in HPMA-Cys) and thioester (in HPMA-ETSA) functionalities. A slight excess of thioester to cysteine groups (molar ratio 3:2) was used to allow further chemical reactions exploiting the unreacted thioester groups. The obtained micelles displayed a Z-average diameter of 80 ± 1 nm (PDI 0.1), and ζ-potential of -4.2 ± 0.4 mV and were linked using two types of pentablock copolymers of P(NIPAM-co-HPMA-Cys)-PEG-peptide-PEG-P(NIPAM-co-HPMA-Cys) (Pep-NC) to yield hydrogels. The pentablock copolymers were synthesized using a PEG-peptide-PEG ATRP macroinitiator and the peptide midblock (lysine-glycine-proline-glutamine-isoleucine-phenylalanine-glycine-glutamine-lysine (Lys-Gly-Pro-Gln-Gly-Ile-Phe-Gly-Gln-Lys)) consisted of either l- or d-amino acids (l-Pep-NC or d-Pep-NC), of which the l-amino acid sequence is a substrate for matrix metalloproteases 2 and 9 (MMPs 2 and 9). Upon mixing of the CCL micelles and the linker (l/d-Pep-NC), the cysteine functionalities of the l/d-Pep-NC reacted with remaining thioester moieties in the micellar core via native chemical ligation yielding a hydrogel within 160 min as demonstrated by rheological measurements. As anticipated, the gel cross-linked with l-Pep-NC was degraded in 7-45 days upon exposure to metalloproteases in a concentration-dependent manner, while the gel cross-linked with the d-Pep-NC remained intact even after 2 months. Dynamic light scattering analysis of the release medium revealed the presence of nanoparticles with a Z-average diameter of ∼120 nm (PDI < 0.3) and ζ-potential of ∼-3 mV, indicating release of core cross-linked micelles upon HyMic exposure to metalloproteases. An in vitro study demonstrated that the released CCL micelles were taken up by HeLa cells. Therefore, HyMic as an injectable and enzyme degradable hydrogel displaying controlled and on-demand release of CCL micelles has potential for intracellular drug delivery in tissues with upregulation of MMPs, for example, in cancer tissues.
中文翻译:

将可注射的MMP降解水凝胶转化为核心-交叉连接的胶束。
在这项研究中,设计并开发了一种新型的可注射水凝胶,称为“ HyMic”,可在暴露于基质金属蛋白酶(MMP)后转化为核心交联(CCL)胶束。HyMic由通过酶可裂解的接头连接的CCL胶束组成。为此,使用原子转移自由基聚合(ATRP)合成了两种以聚乙二醇(PEG)为B嵌段的互补ABA嵌段共聚物。A嵌段由N-异丙基丙烯酰胺(NIPAM)和N-(2-羟丙基)甲基丙烯酰胺-半胱氨酸(HPMA-Cys)或N-(2-羟丙基)甲基丙烯酰胺-乙基硫代乙醇酸琥珀酸(HPMA-ETSA)的无规共聚物组成)。混合获得的聚合物的水溶液,并将温度升高到PNIPAM嵌段的浊点以上,导致这些聚合物自组装成由亲水PEG壳和疏水核组成的花状胶束。胶束核心通过半胱氨酸(在HPMA-Cys中)和硫酯(在HPMA-ETSA中)功能之间的天然化学连接而交联。半胱氨酸基团(半摩尔比为3:2)略微过量的硫酯基可用于利用未反应的硫酯基团进行进一步的化学反应。所得胶束的Z平均直径为80±1 nm(PDI 0.1),ζ电位为-4.2±0.4 mV,并且使用两种类型的P(NIPAM-co-HPMA-Cys)-五嵌段共聚物连接PEG-肽-PEG-P(NIPAM-co-HPMA-Cys)(Pep-NC)产生水凝胶。五嵌段共聚物是使用PEG-肽-PEG ATRP大分子引发剂和肽中间嵌段(赖氨酸-甘氨酸-脯氨酸-谷氨酰胺-异亮氨酸-苯丙氨酸-甘氨酸-谷氨酰胺-赖氨酸(Lys-Gly-Pro-Gln-Gly-Ile-Phe)合成的-Gly-Gln-Lys)由1-或d-氨基酸(1-Pep-NC或d-Pep-NC)组成,其中1-氨基酸序列是基质金属蛋白酶2和9( MMP 2和9)。将CCL胶束和接头(l / d-Pep-NC)混合后,l / d-Pep-NC的半胱氨酸官能团与胶束核心中的剩余硫酯部分通过天然化学连接反应,在160分钟内产生水凝胶如流变学测量所证明。如所预期的,与l-Pep-NC交联的凝胶在暴露于金属蛋白酶后在7-45天内以浓度依赖性方式降解,而与d-Pep-NC交联的凝胶即使在2个月后仍保持完整。对释放介质的动态光散射分析表明,存在纳米颗粒,其Z平均直径约为120 nm(PDI <0.3),ζ电位约为-3 mV,这表明在HyMic暴露于HYMic后释放了核心交联胶束。金属蛋白酶。一项体外研究表明,释放的CCL胶束被HeLa细胞吸收。因此,HyMic作为可注射和可酶降解的水凝胶,显示出可控和按需释放CCL胶束,具有在组织中(例如在癌症组织中)MMP上调的细胞内药物递送的潜力。对释放介质的动态光散射分析表明,存在纳米颗粒,其Z平均直径约为120 nm(PDI <0.3),ζ电位约为-3 mV,这表明在HyMic暴露于HYMic后释放了核心交联胶束。金属蛋白酶。一项体外研究表明,释放的CCL胶束被HeLa细胞吸收。因此,HyMic作为可注射和可酶降解的水凝胶,显示出可控和按需释放CCL胶束,具有在组织中(例如在癌症组织中)MMP上调的细胞内药物递送的潜力。对释放介质的动态光散射分析表明,存在纳米颗粒,其Z平均直径约为120 nm(PDI <0.3),ζ电位约为-3 mV,这表明在HyMic暴露于HYMic后释放了核心交联胶束。金属蛋白酶。一项体外研究表明,释放的CCL胶束被HeLa细胞吸收。因此,HyMic作为可注射和可酶降解的水凝胶,显示出可控和按需释放CCL胶束,具有在组织中(例如在癌症组织中)MMP上调的细胞内药物递送的潜力。
更新日期:2020-01-16
中文翻译:

将可注射的MMP降解水凝胶转化为核心-交叉连接的胶束。
在这项研究中,设计并开发了一种新型的可注射水凝胶,称为“ HyMic”,可在暴露于基质金属蛋白酶(MMP)后转化为核心交联(CCL)胶束。HyMic由通过酶可裂解的接头连接的CCL胶束组成。为此,使用原子转移自由基聚合(ATRP)合成了两种以聚乙二醇(PEG)为B嵌段的互补ABA嵌段共聚物。A嵌段由N-异丙基丙烯酰胺(NIPAM)和N-(2-羟丙基)甲基丙烯酰胺-半胱氨酸(HPMA-Cys)或N-(2-羟丙基)甲基丙烯酰胺-乙基硫代乙醇酸琥珀酸(HPMA-ETSA)的无规共聚物组成)。混合获得的聚合物的水溶液,并将温度升高到PNIPAM嵌段的浊点以上,导致这些聚合物自组装成由亲水PEG壳和疏水核组成的花状胶束。胶束核心通过半胱氨酸(在HPMA-Cys中)和硫酯(在HPMA-ETSA中)功能之间的天然化学连接而交联。半胱氨酸基团(半摩尔比为3:2)略微过量的硫酯基可用于利用未反应的硫酯基团进行进一步的化学反应。所得胶束的Z平均直径为80±1 nm(PDI 0.1),ζ电位为-4.2±0.4 mV,并且使用两种类型的P(NIPAM-co-HPMA-Cys)-五嵌段共聚物连接PEG-肽-PEG-P(NIPAM-co-HPMA-Cys)(Pep-NC)产生水凝胶。五嵌段共聚物是使用PEG-肽-PEG ATRP大分子引发剂和肽中间嵌段(赖氨酸-甘氨酸-脯氨酸-谷氨酰胺-异亮氨酸-苯丙氨酸-甘氨酸-谷氨酰胺-赖氨酸(Lys-Gly-Pro-Gln-Gly-Ile-Phe)合成的-Gly-Gln-Lys)由1-或d-氨基酸(1-Pep-NC或d-Pep-NC)组成,其中1-氨基酸序列是基质金属蛋白酶2和9( MMP 2和9)。将CCL胶束和接头(l / d-Pep-NC)混合后,l / d-Pep-NC的半胱氨酸官能团与胶束核心中的剩余硫酯部分通过天然化学连接反应,在160分钟内产生水凝胶如流变学测量所证明。如所预期的,与l-Pep-NC交联的凝胶在暴露于金属蛋白酶后在7-45天内以浓度依赖性方式降解,而与d-Pep-NC交联的凝胶即使在2个月后仍保持完整。对释放介质的动态光散射分析表明,存在纳米颗粒,其Z平均直径约为120 nm(PDI <0.3),ζ电位约为-3 mV,这表明在HyMic暴露于HYMic后释放了核心交联胶束。金属蛋白酶。一项体外研究表明,释放的CCL胶束被HeLa细胞吸收。因此,HyMic作为可注射和可酶降解的水凝胶,显示出可控和按需释放CCL胶束,具有在组织中(例如在癌症组织中)MMP上调的细胞内药物递送的潜力。对释放介质的动态光散射分析表明,存在纳米颗粒,其Z平均直径约为120 nm(PDI <0.3),ζ电位约为-3 mV,这表明在HyMic暴露于HYMic后释放了核心交联胶束。金属蛋白酶。一项体外研究表明,释放的CCL胶束被HeLa细胞吸收。因此,HyMic作为可注射和可酶降解的水凝胶,显示出可控和按需释放CCL胶束,具有在组织中(例如在癌症组织中)MMP上调的细胞内药物递送的潜力。对释放介质的动态光散射分析表明,存在纳米颗粒,其Z平均直径约为120 nm(PDI <0.3),ζ电位约为-3 mV,这表明在HyMic暴露于HYMic后释放了核心交联胶束。金属蛋白酶。一项体外研究表明,释放的CCL胶束被HeLa细胞吸收。因此,HyMic作为可注射和可酶降解的水凝胶,显示出可控和按需释放CCL胶束,具有在组织中(例如在癌症组织中)MMP上调的细胞内药物递送的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号