当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
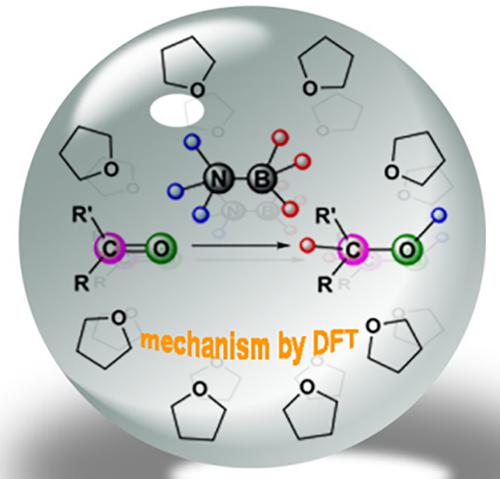
Theoretical insight into the solvent effect on the stoichiometric reduction of carbonyl compounds by ammonia borane and N‐methyl amine borane
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-01-17 , DOI: 10.1002/qua.26162 Nana Ma 1 , Mengxiao Song 1 , Qi Meng 1 , Changgeng Wei 1 , Guisheng Zhang 1
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-01-17 , DOI: 10.1002/qua.26162 Nana Ma 1 , Mengxiao Song 1 , Qi Meng 1 , Changgeng Wei 1 , Guisheng Zhang 1
Affiliation
|
For the traditional reduction of ketones and aldehydes, NH3BH3 (AB) and N‐methyl amine borane (M
nAB) have been effective reducing agents. However, the reaction process is indefinite and different mechanisms have been proposed; also the solvent effect, which is closely related to the mechanism, has not been considered seriously. Here we employ density functional theory to carry out a comprehensive study on the mechanism. The calculated free energy of the concerted double hydrogen transfer process is lower than the hydroboration mechanism by 4.7 kcal/mol, which indicates that reduction of carbonyl by AB is likely due to be the concerted double hydrogen transfer in both aprotic (tetrahydrofuran) and protic (MeOH) solvents. For the reduction by M
nAB, the corresponding free energies of all reactions are higher than those of AB. Meanwhile, the reduction of benzaldehyde by M
nAB (n = 1, 2) also favors a concerted double hydrogen transfer rather than hydroboration.
中文翻译:

溶剂对氨硼烷和N-甲胺硼烷化学计量还原羰基化合物的影响的理论见解
对于传统的酮和醛还原,NH 3 BH 3(AB)和N-甲胺硼烷(M n AB)是有效的还原剂。然而,反应过程是不确定的,并且已经提出了不同的机理。与机理密切相关的溶剂效应尚未得到认真考虑。这里我们运用密度泛函理论对机理进行了全面的研究。一致的双氢转移过程的计算出的自由能比氢硼化机理低4.7 kcal / mol,这表明AB还原了羰基 这可能是由于在非质子传递溶剂(四氢呋喃)和质子传递溶剂(MeOH)中都发生了协同的双氢转移。为了通过减少中号Ñ AB,所有反应的相应的自由能比的更高AB。同时,苯甲醛的由减速中号Ñ AB(Ñ = 1,2)也有利于协同双氢转移而不是氢硼化。
更新日期:2020-03-16
中文翻译:

溶剂对氨硼烷和N-甲胺硼烷化学计量还原羰基化合物的影响的理论见解
对于传统的酮和醛还原,NH 3 BH 3(AB)和N-甲胺硼烷(M n AB)是有效的还原剂。然而,反应过程是不确定的,并且已经提出了不同的机理。与机理密切相关的溶剂效应尚未得到认真考虑。这里我们运用密度泛函理论对机理进行了全面的研究。一致的双氢转移过程的计算出的自由能比氢硼化机理低4.7 kcal / mol,这表明AB还原了羰基 这可能是由于在非质子传递溶剂(四氢呋喃)和质子传递溶剂(MeOH)中都发生了协同的双氢转移。为了通过减少中号Ñ AB,所有反应的相应的自由能比的更高AB。同时,苯甲醛的由减速中号Ñ AB(Ñ = 1,2)也有利于协同双氢转移而不是氢硼化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号