当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Assessing electron transfer reactions and catalysis in multicopper oxidases with operando X-ray absorption spectroscopy.
Nature Communications ( IF 16.6 ) Pub Date : 2020-01-16 , DOI: 10.1038/s41467-019-14210-1 Lucyano J A Macedo 1 , Ayaz Hassan 1 , Graziela C Sedenho 1 , Frank N Crespilho 1
Nature Communications ( IF 16.6 ) Pub Date : 2020-01-16 , DOI: 10.1038/s41467-019-14210-1 Lucyano J A Macedo 1 , Ayaz Hassan 1 , Graziela C Sedenho 1 , Frank N Crespilho 1
Affiliation
|
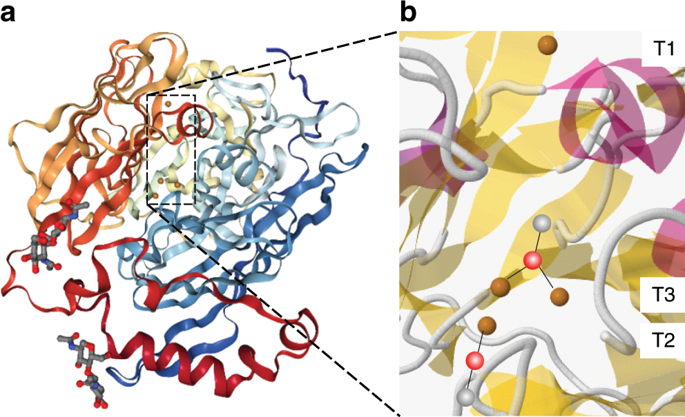
Here we propose an experimental setup based on operando X-ray absorption spectroscopy (XAS) to understand why copper-containing oxidoreductase enzymes show exceptional performance as catalysts for the oxygen reduction reaction (ORR). An electrode based on carbon nanoparticles organized in mesoporous structures with bilirubin oxidase (BOD) was developed to be used in a home-made operando XAS electrochemical cell, and we probed the electron transfer under ORR regime. In the presence of molecular oxygen, the BOD cofactor containing 4 copper ions require an overpotential about 150 mV to be reduced as compared to that in the absence of oxygen. A second electron transfer step, which occurs faster than the cofactor reduction, suggests that the cooper ions act as a tridimensional redox active electronic bridges for the electron transfer reaction.
中文翻译:

用操作X射线吸收光谱法评估多铜氧化酶中的电子转移反应和催化作用。
在这里,我们提出一种基于操作X射线吸收光谱(XAS)的实验装置,以了解为什么含铜的氧化还原酶作为氧还原反应(ORR)的催化剂表现出优异的性能。开发了一种基于碳纳米粒子的电极,该碳纳米粒子以中孔结构与胆红素氧化酶(BOD)组织在一起,被用于自制的操作XAS电化学电池中,并研究了ORR体制下的电子转移。在存在分子氧的情况下,与不存在氧的情况相比,含有4个铜离子的BOD辅因子需要降低约150 mV的超电势。第二个电子转移步骤比辅因子还原快,它表明铜离子充当电子转移反应的三维氧化还原活性电子桥。
更新日期:2020-01-16
中文翻译:

用操作X射线吸收光谱法评估多铜氧化酶中的电子转移反应和催化作用。
在这里,我们提出一种基于操作X射线吸收光谱(XAS)的实验装置,以了解为什么含铜的氧化还原酶作为氧还原反应(ORR)的催化剂表现出优异的性能。开发了一种基于碳纳米粒子的电极,该碳纳米粒子以中孔结构与胆红素氧化酶(BOD)组织在一起,被用于自制的操作XAS电化学电池中,并研究了ORR体制下的电子转移。在存在分子氧的情况下,与不存在氧的情况相比,含有4个铜离子的BOD辅因子需要降低约150 mV的超电势。第二个电子转移步骤比辅因子还原快,它表明铜离子充当电子转移反应的三维氧化还原活性电子桥。



























 京公网安备 11010802027423号
京公网安备 11010802027423号